CH 610B
Second Exam
Spring,2002
Prof.N.L.Bauld
__________________________________________ Name (Last,
First)
__________________________________________ Social Security Number
Question |
Possible Points |
Page/Pts Earned |
Score |
|
I. |
22 |
2/11/ 3/11/ |
|
|
II. |
23 |
4/12/ 5/11/ |
|
|
III. |
19 |
6/9/ 7/10/ |
|
|
IV. |
15 |
8/7/ 9/8 |
|
|
V. |
23 |
10/10/ 11/8/ 12/5/ |
|
|
Totals |
102 |
|
|
I. Nuclear Magnetic Resonance (NMR)
A.The Basic Experiment
1.
[3 pts] Assume a nucleus which has two spin states, designated as a and b Draw a diagram of the
energies (E) of these two spin states on the Y axis vs. H (on the X axis) as a
large, external magnetic field (H) is applied. Explain the qualitative effect
of H upon each of these spin states.
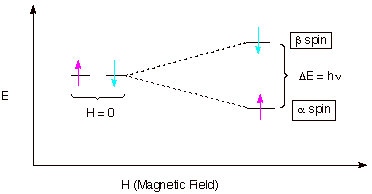
q
The two spin states
are of equal energy in the absence of the magnetic field.
q
When the field is
applied, the alpha state, which is the state having the spin in the same
direction as the applied field, decreases in energy, while the beta state,
which has its spin opposed to the applied field increases in energy.
q
The energy difference
increases as the magnitude of the field increases.
2.
[2 pts] What is the requirement, expressed in terms of a mathematical equation,
for such a nucleus to absorb radiation of frequency n? What happens to the spin state when this radiation is absorbed?
q
DE = hn.
q
The spin state which
absorbs the radiation is converted into the other spin state.
3.
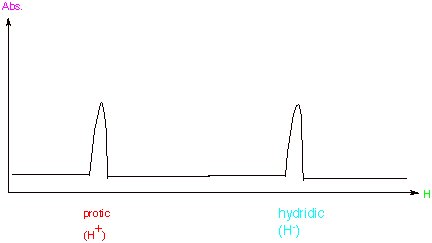
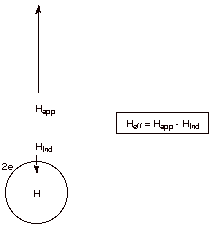
[4 pts] Consider two extreme electronic forms of the hydrogen nucleus, i.e.,
the proton (H+) and the hydride ion (H-). Show on a plot
of absorption (A) vs. magnetic field (H) qualitatively where these species
would absorb relative to each other (high field or low field). Explain, with
the assistance of another diagram, why a larger magnetic field is required to
reach resonance for one of these species than the other. What is the name for
this kind of magnetic effect?
q
In the presence of
the large external magnetic field, the paired electrons around the nucleus of
the hydride anion set up an induced magnetic field which is opposed to the
direction of the applied field, thus making the net field at the nucleus less
than the value of the applied field. Consequently, a larger external applied field is needed to satisfy the resonance
condition than in the case of a bare proton, which has no electrons around it.
q
This is called diamagnetic
effect.
B. Anisotropic Effects
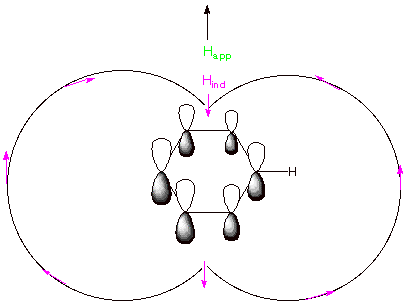
1.
[2 pts] Explain, with the assistance of a diagram, why the hydrogen nuclei of
benzene or other aromatics absorb at very low fields. What is the approximate
chemical shift of such a proton?
2.
[3 pts] What is the approximate chemical shift of an aldehydic proton? Explain
this chemical shift qualitatively in terms of two distinct effects and give the
names of the effects.

q
The carbonyl carbon has
a large partial positive charge, so that carbon is electronegative and draws
electron density from the aldehydic hydrogen, making it more protonic. This is
an electronegativity effect.
q
The carbonyl pi bond,
like an alkene pi bond, has a diamagnetic effect which arises from the pi
electrons, and shifts protons which are in the trigonal plane downfield.
q
The two effects combine
to shift these protons to the very downfield region.
C. Simulated NMR Spectra
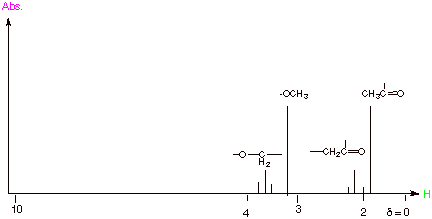
1.
[6 pts] Provide a simulated NMR spectrum for the following molecule, mapping
the spectrum from d = 0 to 10 or a smaller segment of the spectrum as
needed. For each different set of protons (1) indicate which proton or group of
protons in the given molecule give rise to this absorption (2) explain the
chemical shifts of each set relative to
typical aliphatic methyl absorption at d = 0.9 in
terms of an electronic effect, and
finally (3) explain the multiplicity of each set of protons, indicating what
rule you are using to explain the multiplicities.
![]()
q
The methyl and
methylene groups attached to the carbonyl group are shifted downfield from
aliphatic methyls by an electronegativity effect, since the attached carbonyl
carbon is electron withdrawing.
q
The methoxy and
methyleneoxy groups are shifted even more downfield because the oxygen atom
attached to these carbons is highly electronegative.
q
The methyl attached
to the carbonyl group is a single, because there is no vicinal hydrogen; the
methoxy protons also are a singlet, since there are no vicinal protons on the
neighboring oxygen; The two methylene groups are triplets as a result of the
2n+1 rule, i.e., each methylene group is split into a triplet because there are
two vicinal protons (n=2).
q
It is important that
the methyl and methylene protons attached to the carbonyl group are shown as
absorbing at ca.
d =2, but the sequence of these
two is not important. Similarly, the methylene and methyl groups which are
attached to oxygen should be shown as absorbing at ca. d = 3.5-4.0, but the sequence (up or down field is not crucial
for this exam.
D. Calibration of Chemical
Shifts.
1.
[2 pts] What molecule is used to calibrate the reference point d = 0? Why is this molecule particularly appropriate (at least two
reasons)?
q
Tetramethylsilane (Me4Si)
q
The TMS protons are
somewhat hydridid because silicon is electropositive relative to carbon. These
protons are therefore more upfield than most protons in strictly organic
molecules.(This answer is required)
q
Also, TMS is a
relatively unreactive and stable molecule, so it is compatible with most
organic compounds.(Unreactive or stable is acceptable as the second answer.
II.Enols and
Enolates
A. Thermodynamic Stability of Enols
1.
[2 pts] Which is more stable, the keto form or the enol form shown below.
Explain the basis for your conclusion in terms of bond strengths.
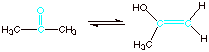
q
The ketone form is
more stable than the enol form, because the carbonyl double bond is much more
thermodynamically stable than the alkene double bond present in the enol.
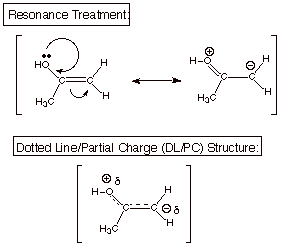
2.
[4 pts] Provide a resonance theoretical treatment of the enol shown above and
summarize as a dotted line/partial charge (DL/PC) structure. Is the enol double
bond more or less reactive toward electrophiles (such as H+) than a
simple alkene double bond? Explain in terms of a character revealed by your
DL/PC.
The enol double bond is
much more reactive toward electrophiles because it has a substantial partial
negative charge accumulation, i.e., it has carbanion character.
B. Mechanism of Acid
Catalyzed Enolization
1.
[3 pts] Write the detailed mechanism for the acid catalyzed enolization of
acetone (the ketone form shown in the above question) in aqueous solution
containing strong acid (hydronium ion).
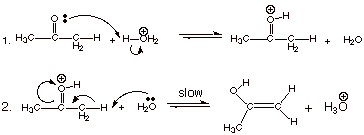
C. Mechanism of Base
Catalyzed Enolization
1.
[3 pts] Write the detailed mechanism for the base catalyzed enolization of acetone.
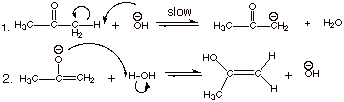
**Note that either
canonical form (or both) of the enolate can be written here.
D. Acidity of Carbonyl
Compounds
1.
[2 pts] What is the approximate pKa value of a simple ketone like
acetone. Is acetone more or less acidic than water? What is the pKa
of water?
![]()
q
The approximate pKa
is 19 (accept 18-20)
q
Acetone is therefore
less acidic than water.
q
Water has a pKa
of about 16 (15.7).
2.
[2 pts] Provide a resonance theoretical treatment of the acetone enolate,
summarize as a dotted line/partial charge structure, and characterize this
structural model.
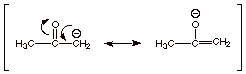
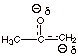
q
Oxyanion (alkoxide)
character
q
Carbanion character
3.
[3 pts] Referring to your resonance structures for the enolate, indicate a
factor which favors each of the canonical structures (one factor favoring the
structure with negative charge on carbon and one favoring the structure with
negative charge on oxygen). What is the relevance of these counteracting
factors to the magnitude of the resonance stabilization?
q
The structure with
oxyanion character tends to be favored because oxyanion character is more
favorable than carbanion character.
q
Thes structure with
carbanion character tends to be favored because it has a carbonyl double bond, which is more stable than the
alkene double bond present in the oxyanion structure.
q
These factors are
relevant in that they tend to oppose and cancel one another, bringing the two
structures very close together in energy, a circumstance which maximizes the
resonance stabilization of the enolate.
4.
[4 pts] Consider the acid/base reaction below and use a qualitative criterion
to predict whether the equilibrium goes to the product or the reactant side.
State the criterion you are using. Then, state and apply a quantitative
criterion to obtain the actual equilibrium constant for this acid/base
reaction.
![]()
q
Qualitative
Criterion: “The stronger acid is converted to the weaker acid.” The
stronger acid is acetone, which has a pKa of ca. 19, while the
weaker acid is NH3 , which has a pKa of 38.
Thereforfe the reaction goes to the right as written.
q
Quantitative
Criterion: K = Ka (Reactant Acid)/Ka (Product Acid)
K = 10-19/10-38 =
1019
Therefore
the reaction goes far to the right, specifically with the K value shown.
III. Aldol Addition
and Condensation
A.Mechanism of the Aldol Addition Reaction
1. [4 pts] Write the detailed mechanism for the base
catalyzed aldol addition reaction of propanal. Provide the IUPAC name and the
structure of this product.
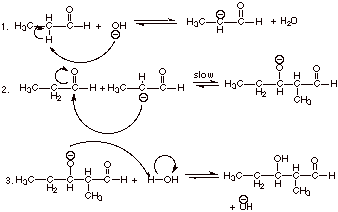
q
The IUPAC name of the
aldol product is 3-hydroxy-2-methylpentanal.
2. [3 pts] Provide the structures of 2 aldehydes which do not undergo the aldol reaction and explain why.
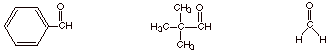
q
These three aldehydes
(only two are required) exemplify aldehydes which do not have an alpha hydrogen
and therefore cannot form an enolate, which is required in the aldol reaction.
(Note that the last one doesn’t even have an alpha carbon).
B.Crossed Aldol Reactions.
1.
[2 pts] Explain why the crossed aldol reaction between ethanal and propanal is
not synthetically useful.
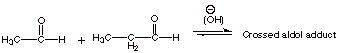
q
There are two
different enolates (one from each aldehyde) and two different carbonyl
components (aldehydes). Therefore, there are four different aldol products, all
of which would be formed competitively.
2.
[3 pts] Draw the structure of the product of the following successful crossed
aldol reaction and explain why this reaction works efficiently. Which reactant
should be added last and in a dropwise manner? Explain why this is important in
obtaining good yields of the crossed aldol adduct.
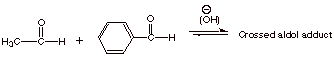
![]()
q
Only acetaldehyde
(ethanal) can form an enolate, so there is only one enolate which can be
formed. The cross product must be that of this enolate with benzaldehyde as the
carbonyl component, as shown.
q
Acetaldehyde should
be added last, so that the concentration of benzaldehyde is always in excess. This
is so that the enolate of acetaldehyde will prefer to add to benzaldehyde
(present in excess) over addition to acetaldehyde, which would give the regular
aldol product of acetaldehyde.
C.Mechanism of the Aldol
Condensation Reaction
1.
[2 pts] Write the detailed mechanism for the second stage of the aldol
condensation reaction, given in the equation below:
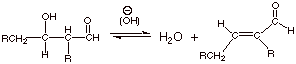
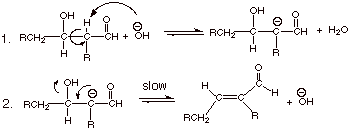
2.
[1 pts] What is unusual about the leaving group in this reaction?
q
The leaving group is
hydroxide anion, which is not a really good leaving group.
3.
[1 pts] Is this elimination mechanism of the E2 type? Explain why or why not.
q
No, this mechanism is
not of the E2 type. The elimination is not concerted, like an E2 mechanism
would be. Instead, it occurs in a stepwise manner, via the intermediate
enolate.
D. Intramolecular Aldol
Reaction
1.
[3 pts] Draw the structure of the enolate which would be formed from the
following difunctional aldehyde in aqueous base, and indicate by electron flow
arrows how it would react intramolecularly (within the same molecule). Draw the
structure of the aldol addition product. What is unique about the structures of
the products of the intramolecular
aldol reaction.
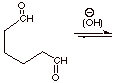
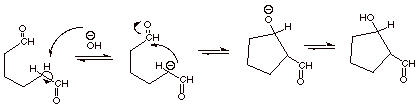
IV. Claisen Ester
Condensation; Acetoacetic and Malonic Ester Syntheses
A.Mechanism of the Claisen
Ester Condensation
1.
[3 pts] Write the detailed mechanism for the Claisen ester condensation of
ethyl acetate. You may use EtO- and EtOH to abbreviate ethoxide and
ethanol. Similarly, you may use Et in the ester to abbreviate the ethyl group.
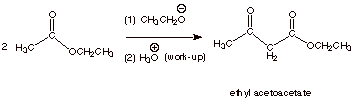
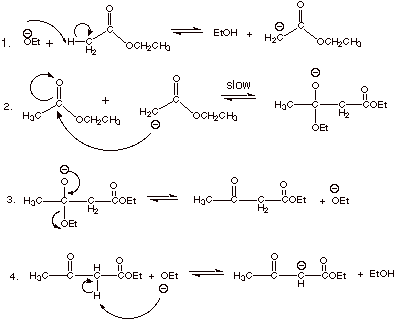
2.
[2 pts] Is the conversion as written above, i.e., the conversion of two
molecules of ethyl acetate to one of ethyl acetoacetate (plus one molecule of
ethanol) energetically favorable? To put it another way, is the result of the
first three steps of your mechanism (if written correctly) a favorable
conversion? Why or why not? Why does the Claisen condensation succeed?
q
The conversion of an
ester functionality to a ketone functionality is unfavorable energetically,
because of the resonance stabilization of the ester function. Therefore the
result of the first three steps is not favorable for the reaction.
q
The Claisen
condensation succeeds because the last step, a highly exergonic acid/base
reaction, provides the thermodynamic driving force.
3.
[2 pts] Provide a resonance theoretical treatment of the conjugate base of ethyl acetoacetate, and summarize it as
a dotted line/partial charge (DL/PC) structure.
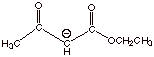
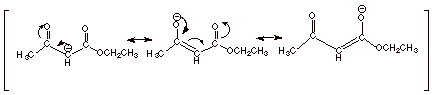
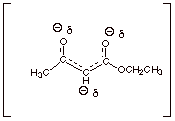
4.
[2 pts] What is the pKa of ethyl acetoacetate? Of Ethyl acetate?
Explain the difference.
q
The pKa of
ethyl acetoacetate (acetoacetic ester)is ca. 10.
q
The pKa of
ethyl acetate is ca. 21.
q
The enolate of
acetoacetic ester is more highly resonance stabilized than that of ethyl
acetate, because the former has three resonance structures, while the latter
has only has two. (To put it differently, the negative charge of the enolate of
ethyl acetoacetate is delocalized over a more extended conjugated system than
that of ethyl acetate.
5.
[1 pts] Provide an estimate of the pKa of the ester shown below and
explain the basis for your prediction.
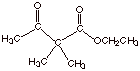
q
The pKa is
about 19, that is about that of an ordinary ketone which has alpha hydrogens.
q
The carbon atom which
is between the two carbonyl groups has no alpha type hydrogens attached, so it
does not have the extra acidity of beta dicarbonyl compounds.
B. Acetoacetic Ester
Syntheses
1.
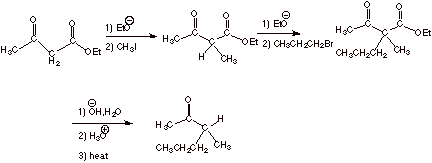
[4 pts] Sketch a synthesis of the following ketone using ethyl acetoacetate and
any alkyl halide having 4 carbons or less.
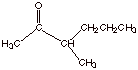
. Aromaticity
A.Aromaticity of Benzene
1. One canonical structure for benzene is illustrated below. What is the name given to this structure of benzene?
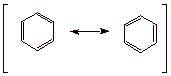
Kekule structure
2.
In the space provided above in question V.A.1, complete the usual resonance
theoretical treatment of benzene , and indicate in the space below this what
are the energetic and geometric results of this resonance stabilization. Be as
quantitative and specific as you can about both the energetic and geometric
consequences of benzene’s resonance stabilization.
q
Benzene is highly
resonance stabilized, i.e., its energy is lowered by at least 36 kcal/mol
compared to cyclohexatriene.
q
Instead of having
alternate double and single bonds, benzene is a regular hexagon, with all bonds
being equivalent and 1.39 A long.
3.
Explain in terms of the molecular orbital picture of the pi electronic system
of benzene provided below, why benzene is aromatic (strongly resonance
stabilized). What is the significance of the dashed line? What are the
MO’s which fall below the dashed line called? Those which appear above
the dashed line?
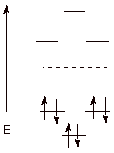
q
The dashed line
represents the non-bonding energy leve,i.e., the energy of an electron in an
atomic orbital (2p) on carbon.
q
Orbitals which are
more stable (below the non-bonded line) are called bonding molecular orbitals
(BMO’s).
q
Orbitals which are
less stable (above the NB line) are called antibonding molecular orbitals
(ABMO’s).
q
Benzene is so highly
resonance stabilized because there are three strongly bonding MO’s and
the six electrons in the pi system of benzene fill all three of the BMO’s
and none of the ABMO’s. [Give +1 extra credit if it is mentioned that the
most bonding MO in cyclic systems is especially low in energy]
4.
Using the mnemonic device we developed in class (called the Frost-Musulin mnemonic)
provide a similar diagram of the MO’s of the pi electron system of cyclobutadiene. Is this
system aromatic? Explain why or why not. (Do not use the Huckel rule to explain
this.)
![]()
1,3-cyclobutadiene (one canonical structure)
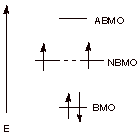
q
The MO’s follow
the pattern of a square, with one vertex down. This means there is only one BMO. The next two are at the non-bonding level.
q
The four electrons of
the two double bonds (formally) of cyclobutadiene cannot all go into bonding
orbitals. Two of them go into non-bonding orbitals (NBMO’s)which provide no molecular bonding.
q
Consequently,
cyclobutadiene is antiaromatic (or
non-aromatic).
q
[1 point extra credit if
it is mentioned that the electrons go into different NBMO’s with their spins unpaired, according to the Hund Rule.
5. The molecule shown below is called pyridine. Is pyridine aromatic or not? Explain why or why not, including in your explanation the unshared pair on nitrogen. Also, draw an orbital picture of the pi system of pyridine, showing the overlap of the 2p AO’s and also the orbital occupied by the unshared pair on nitrogen.
![]()
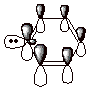
q
Pyridine is aromatic. It
has a six orbital, 6 electron pi system just like benzene.
The unshared pair is in an sp2
AO on nitrogen, which is perpendicular to the 2pz AO’s which
form the aromatic system, and are not a part of the pi system.
B. Systems Other Than
Benzene.
1.
Classify the conjugated pi electron systems shown below as aromatic or
antiaromatic (or, if you prefer, non-aromatic), explaining your answer and
showing how you are counting electrons.
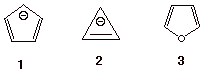
q
The cyclopentadienyl
anion (1) is aromatic. It
has a pi system containing 6 electrons, which conforms to the requirements of
the Huckel 4n+2 rule, with n=1. .The electrons are counted as follows: Two
electrons for each of the double bonds (total of 4), and two electrons for the
carbanion center.
q
The cyclopropenyl
anion (2) is antiaromatic,
since it has 4 electrons (4n). These come from one double bond and one
carbanion center.
q
The third molecule,
furan, is aromatic. The divalent oxygen has two unshared pairs. One of them is
in the trigonal plane and not a part of the pi system, but the other is in the
pi system. These two electrons make the electron count 6.
C. Phenol
1.What
is the approximate pKa of phenol? Compare this to the pKa
of an ordinary alcohol. By what factor is phenols acidity increased or
decreased? Which is it, increased or decreased?

q
pKa of
phenol is ca. 10.
q
pKa of typical alcohols is ca. 16.
q
The acidity of
phenols is greater by 106 (one million times).
2. Using resonance theory, explain the sharp difference
in acidities of ordinary alcohols and phenols.
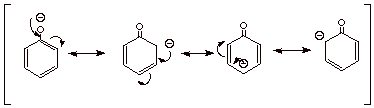
q
The phenoxide anion,
the conjugate base of phenol, is highly resonance stabilized, with the negative
charge being delocalized onto the ortho and para positions of the benzene rin,
as shown in the four canonical structures above.
q
This makes the
phenoxide anion much more thermodynamically stable than an alkoxide anion, the
conjugate base of an ordinary alcohol. Consequently, the acid dissociation
equilibrium for phenol lies much farther to the right, producing much more
hydronium ion.
3.
What base can be used to separate phenols from carboxylic acids and ordinary
alcohols by means of acid/base extraction techniques? Explain, in terms of the
qualitative theory which allows one to predict when an acid/base reaction will
go to completion, why this method works.
q
Bicarbonate is basic
enough to neutralize a carboxylic acid, but not enough to neutralize phenol.
The carboxylate anion is thus extracted into the aqueous phase as the sodium
salt, while the phenol remains soluble in the organic phase.
q
This works because
the stronger acid in an acid/base reaction goes to the weaker acid (see below).
Carbonic acid, the conjugate acid of bicarbonate anion, is a stronger acid than
phenol, so the equilibrium favors the weaker acid, phenol. On the other hand,
carboxylic acids (pKa ca. 5) are stronger acids than carbonic acid
(pKa ca. 7), so carboxylic acids are converted to carboxylate anions
and carbonic acid.
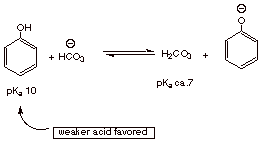
4.
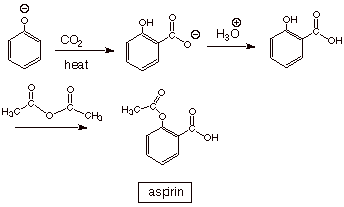
Sketch an efficient synthesis of aspirin.