CH 610B
Third Exam
Spring,2002
Prof.N.L.Bauld
__________________________________________ Name (Last,
First)
__________________________________________ Social Security Number
Question |
Possible Points |
Page/Pts Earned |
Score |
|
I. |
28 |
2/6/ 3/13/ 4/9/ |
|
|
II. |
24 |
5/8/ 6/10/ 7/6/ |
|
|
III. |
14 |
8/8/ 9/6/ |
|
|
IV. |
24 |
10/10/ 11/8/ 12/6/ |
|
|
V. |
12 |
13/12 |
|
|
Totals |
102 |
|
|
I. Electrophilic Aromatic Substitution
A. General Considerations
1.
[3 pts] Write the detailed mechanism for the generalized electrophilic aromatic
substitution reaction shown below (use just one canonical structure for the
intermediate).
![]()
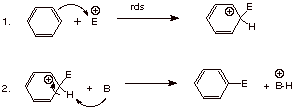
[Use any base B or B-]
2.
[3 pts] Provide a resonance
theoretical treatment of the intermediate arenium ion, summarize as a dotted line/partial charge structure, and
characterize the intermediate as carefully as you can (where is the character
and where isn’t it?).
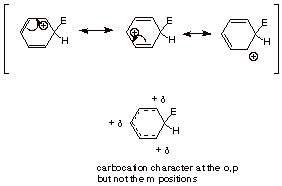
Note: No dotted lines between
the ipso and ortho positions.
4.
[5 pts] Using resonance theory, develop a TS model
for the rate-determining step of
this same generalized reaction, summarize as a DL/PC structure, and characterize this model.Finally, apply the Hammond principle to
the model.
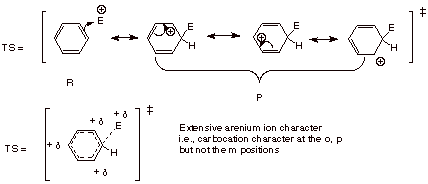
q
The reaction step is
endergonic, i.e., energetically uphill, so that the TS more closely resembles
the product of that step, namely, the arenium ion. So the TS has extensive
arenium ion character, which means extensive carbocation character at the o and
p positions, but not the m position.
5. [2 pts] What effect would the resonance stabilization of the arenium ion (or more accurately, the arenium ion character of the TS), taken alone, have on the rate of benzene’s reaction with an electrophile? Explain why the reaction of benzene with an electrophile is much slower than the corresponding reaction of ethene with the same electrophile.
q
The resonance
stabilization of the TS should lower the activation energy and accelerate the rate.
q
Actually, the rate of
reaction of benzene with electrophiles is much slower than
that with ethene. The reason is that the resonance stabilization of the
reactant, benzene tends to increase the activation energy. The resonance stabilization of benzene
is much greater than that of the arenium ion, so the net effect is deceleration.
B.Nitration of Benzene.
1. [4 pts] Write the detailed
mechanism for the nitration of benzene in mixed acid.
![]()
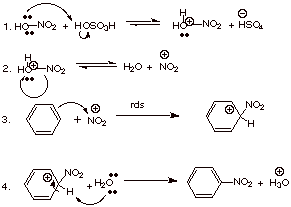
[alternatively, you may use
the bisulfate anion as the base: HSO4-].
3.
[2 pts] Given that the
“attack step” is rate determining, what does this tell us about the
reversibility of this step? Explain why it is plausible that this step is not
reversible.
No step that is reversible can be rate determining, so this rds is not reversible. It is plausible because it is easier to lose a proton—step 4—that a nitronium ion—reverse of step 3.
C. Friedel-Crafts
Acylation.
1.
[3 pts] Write the detailed
mechanism for the Friedel-Crafts reaction of benzene with acetyl chloride, as
shown in the illustration below.
![]()
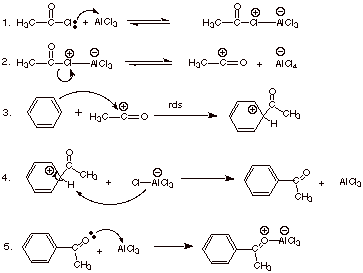
2.
[2 pts] Provide a resonance theoretical treatment of the active electrophile
(the species which actually attacks the benzene ring) in this reaction,
summarize it as a DL/PC structure and characterize it.
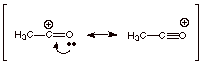
![]()
q
Note: The DL/PC
structure should have two full C-O bonds and one partial. My graphics program
always converted the dashed,
partial bond to a full one.
q
The structure has
carbocation and oxonium ion character.
3.
[2 pts] Although this reaction is an electrophilic substitution on benzene, it is a nucleophilic substitution on the acetyl chloride, specifically at the carbonyl
carbon. Is it an SN2 or SN1 reaction? Explain
why this particular mechanism is favored.
q
It is an SN1
reaction, since the benzene nucleophile is not involved in the step where the
leaving group is lost—a carbocation is formed.
q
It is favored because
the carbocation involved is strongly resonance stabilized.
4.
[2 pts] What quantity of aluminum
chloride must be provided for this reaction to go in high yield? Explain the
reasons for this in detail, indicating a specific Lewis acid/Lewis base
reaction as the basis for your answer.
q
Stoichiometric
amount. Because the AlCl3 is consumed by irreversible
combination with the Lewis basic ketonic oxygen of the product, as shown in the
mechanism.
II. Rates and Selectivity in
Electrophilic Aromatic Substitution Reactions
A. Relative Rates.
1.
[4 pts] Use the Method of Competing Transition States to discuss relative rates in electrophilic
substitutions of a general electrophile (E+) with benzene and
toluene (methylbenzene). Specifically, compare the dotted line/partial charge
(DL/PC) TS models for the reaction with benzene with the corresponding DL/PC for reaction at the para
position of toluene. (You may use the
generalized TS model developed in question I for the reaction of the
electrophile with benzene, repeating it in the space below, and then comparing
it with the analogous TS for the corresponding reaction at the para position of
toluene; You do not have to derive these TS models in this question). Indicate which
TS is favored and why, and then which rate is faster.
TS for the Reaction with
Benzene vs. TS for the Reaction at p-position of Toluene
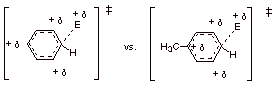
q
The toluene TS is
favored because the methyl group is attached to a carbon which has carbocation
character. The methyl group stabilizes carbocation character.
q
Therefore, the
reaction with toluene is faster than that with benzene.
2.
[1 pts] Is the relative rate ratio likely to be large or small? Explain your
answer based upon refined TS character.
q
The relative rate
ratio is relatively large, because there is extensive carbocation character at the para position, the
position to which the methyl group is attached.
B. Selectivity (Orientation).
1.
[3 pts] Again using the Method of Competing TS’s, compare the DL/PC TS
models for para vs. meta substitution
in toluene by a general electrophile. (You do not have to derive these TS’s). Indicate which is favored and why. Show the
structures of the two main products of this reaction.
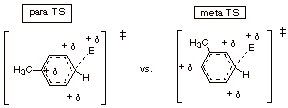
q
The para TS is favored because
there is carbocation character on the attached, para, carbon, which the methyl
group will be able to provide stabilization for. In the meta TS, there is no
carbocation character at the meta carbon, and so no large resonance
stabilization of the carbocation character.
q
The two main products
are the ortho and para ones.
![]()
2.
[3 pts] Arrange the three carbocation structures shown below in order of
decreasing stability (most stable = 1), Explain why your structure rated #1 is
more stable than your structure rated #3 (the least stable one), using
resonance theory and showing resonance structures. Then, explain why structure
#1 is more stable than structure #2, again using resonance theory. For the
purposes of discussing substituent effects, what is the name given to groups
which stabilize a carbocation center?
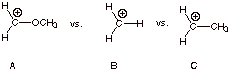
1 3 2
q
A is resonance stabilized, but B is not.
q
Both A and C are
resonance stabilized, but the methoxy group provides more stabilization than
the methyl group because it has a non-bonded electron pair to delocalize, while
the methyl group has a bonded (more stable) electron pair from a C-H bond. [Or:
you could say that 1 is stabilized
by conjugation and 2 by
hyperconjugation.
q
The methoxy and methyl
groups are both electron donating groups (EDG’s).
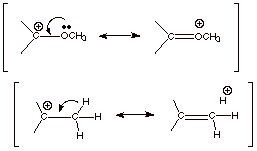
3.
[3 pts] Consider the effect of the chlorine substituent on the carbocation
structure shown below. Provide the names of two different effects by which the chlorine substituent affects the
carbocation’s stability, illustrate
these effects pictorially, and indicate
whether each effect tends to stabilize or destabilize the carbocation. Finally, indicate which effect is
dominant, and to what class of
substituents the halogens belong as a
result of this dominance.
![]()
q
Cl stabilizes a directly
attached carbocation center by means of a resonance effect:
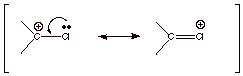
q
Cl destabilizes a
carbocation center by an inductive (I ) effect derived from the sigma bond
dipole.
![]()
q
The destabilizing I
effect is dominant, so that Cl is an electron withdrawing group (EWG).
4.
[2 pts] Now, consider the
carbocation structure A in the
preceding question. Indicate which effect is dominant in this case, and explain
the contrast between this case and that of the chlorine substituent.
q
In this case the
resonance (R) effect is dominant because the electron pair on oxygen is more
readily available for delocalization than that on chlorine.
5. [2 pts] Although Cl, Br, and I substituents are net electron withdrawing, the most electronegative substituent of all, F, is found often to be weakly electron donating. Explain this contrast between Cl and F in terms of a resonance effect, using a structural depiction to illustrate your answer.
![]()
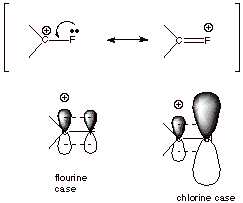
q
In the case of F, the
carbon and fluorine 2p AO’s are of very similar size, so that overlap is
efficient, and resonance stabilization optimized. In the case of Cl, there is a
size mismatch with the larger Cl 3p AO, so that overlap is inefficient and
resonance stabilization is much less.
III. Amines
A. Nomenclature
1.
[2 pts] Draw the structure of any
tertiary amine and name the amine using IUPAC nomenclature.
![]()
q
As one example. If
the still simpler trimethylamine is used, the correct name is:
N,N-dimethylmethanamine (not trimethylamine).
B. Basicity
1.
[4 pts] Consider the reaction of an amine with a carboxylic acid,
as illustrated below. Given the approximate pKa’s of the two
acids involved in the equation, use two different approaches to determine
whether this reaction goes to the right or to the left. In the first case, use
a qualitative criterion, stating
the criterion you are using and explaining the specific application of the
criterion to this equation. In the second case, use a quantitative criterion, specifying the general equation for obtaining the
equilibrium constant for this reaction and showing your work in deriving the
equilibrium constant, K, for this reaction. Again, does this equilibrium
constant indicate the reaction goes to completion for practical purposes?
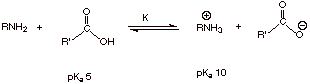
Qualitative Criterion:
q
The stronger acid is
converted to the weaker acid.
q
Acetic acid, with a pKa of 5,
is a stronger acid than the alkylammonium ion, with a pKa of 10, so
the reaction goes to the right as written.
Quantitative Criterion:
q
The equation for the
equilibrium constant, K, of an acid base reaction is given by:
![]()
q
Therefore the reaction
goes far to the right, since the K value is much greater than 1.
K
= 10-5/10-10 = 105
2.
Given the following pKa values for the conjugate acids of the indicated amines:
![]()
a. [1 pt] Indicate which amine is the strongest base and
explain your reasoning based upon the pKa values given (not based
upon structural effects).
q
Methylamine is the
strongest base, since its conjugate acid is the weakest acid.
b. [3 pts] Is methylamine or ammonia the stronger
base? Explain, based upon a substituent effect, giving the name of the effect and a structural illustration of the effect. Is a resonance effect operative? Why
or why not?
q
Methylamine is a
stronger base than ammonia.
q
The methyl group
stabilizes the conjugate acid of ammonia by an inductive effect, making it
easier to protonate.
![]()
q
No, a resonance
effect is not operative, since N cannot expand its valence to 5. (see invalid
resonance structure).
![]()
c. [2 pts] Is trimethylamine a stronger or weaker base
than methylamine? Again, explain this basicity sequence, providing the name of
the effect and a clear structural illustration of the effect.
q
Trimethylamine is a
weaker base than methylamine.
q
Steric hindrance of
solvation of the conjugate acid is the cause.
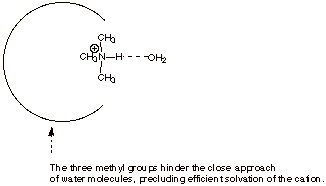
d. [2 pts]
The pKa of pyridine is ca. 5. Is this a stronger or weaker
base than the amines in the above list? Explain in detail. Does this have
anything to do with the aromaticity of pyridine? Explain why or why not.
![]()
q
Since the conjugate
acid of pyridine is evidently a stronger acid than that of the aliphatic amines
above, pyridine must be a weaker base than these amines.
q
It has nothing at all
to do with the aromaticity of pyridine,which is retained in the conjugate acid
since the unshared pair and the accepted proton are in the trigonal plane.
q
The reason is that
the unshared pair of electrons is in an sp2 AO in pyridine, which is
an orbital of lower energy. It is therefore more difficult to protonate.
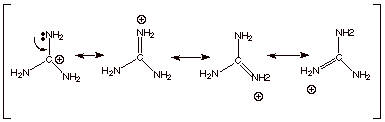
e. [2 pts] Guanidine is an exceptionally strong amine
base. Explain, providing the resonance structures of the conjugate acid.
![]()
q
The conjugate acid of
guanidine has 3 equivalent resonance structures, along with one other canonical
structure, and is therefore rather highly resonance stabilized. This makes it
very easy to protonate.
C. Synthesis
1.
[2 pts] Sketch an efficient synthesis of the following amine, using
1-bromobutane as a starting material:
![]()
![]()
2.
[2 pts] Explain why the following
projected synthesis may be problematic.
![]()
q
The first step,
giving the primary amine, as shown above, works, but this amine can also react
with bromobutane to give dibutyl amine, and so on until tributyl amine, dibutyl
amine, and some butylamine are formed. So a mixture of primary, secondary, and
tertiary amines is formed.
IV. Aromatic Amines/ Hoffmann Eliminations
A. Aniline
1.
[3 pts] Provide a resonance theoretical treatment of aniline and summarize as a
dotted line/partial charge structure. What qualitative effect would the
resonance stabilization of aniline have upon its basicity? Explain.

q
Stabilization of the
amine shifts the equilibrium to the left, giving less base. So resonance
stabilization decreases the acidity of aniline.
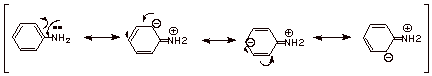
2.
[2 pts] Consider the anilinium ion, the conjugate acid of aniline. Comment on
the resonance stabilization of this cation and explain why it is or is not
resonance stabilized (do not consider the aromaticity present in the ring).
What effect would any resonance stabilization of the anilinium ion have upon
the basicity of aniline?
q
Stabilization of the
conjugate acid would make make aniline a stronger base, by shifting the
equilibrium to the right.
q
The conjugate acid,
anilinium ion, is not
resonance stabilized, since moving a pair of electrons toward nitrogen would
require it to expand its valency.
3.
[2 pts] Is aniline a weaker or stronger base than a primary aliphatic amine
such as methylamine? Explain, using the concept of resonance stabilization and
considering both aniline and anilinium ion.
q
Aniline is a much
weaker base than primary aliphatic amines because aniline is resonance
stabilized and its conjugate acid is not.
B.Synthesis of Aniline
1.
[2 pts] Sketch an efficient synthesis of aniline starting with benzene and any
inorganic reagents and solvents needed.
![]()
![]()
C. Diazonium Ions
1.
[1 pt] Provide, over and under the reaction arrow, the conditions needed to
convert aniline to the corresponding benzenediazonium ion. Write two resonance
structures for the diazonium ion.
![]()
q
Reagents/conditions
needed: HNO2, 0 – 5o C; or instead of HNO2,
use NaNO2, HCl.
2.
[2 pts] Predict the structure of the product formed in the following reaction.
What kind of reaction is this mechanistically? What is the leaving group?
q
Product is iodobenzene.
q
Mechanism is SN
: Preferably SN1 but count either this or SN2.
q
The leaving group is
nitrogen (dinitrogen).
![]()
![]()
D. Azo Compounds
1.
[2 pts] Sketch the mechanism for
the reaction of benzenediazonium ion with N,N-dimethylaniline, providing the
structure of the product. Cite and explain anything unusual about the rate
determining step of this reaction
![]()
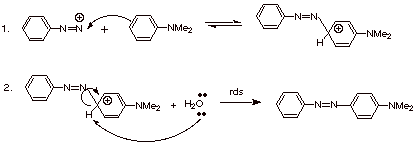
[since the question called for a sketch, we will not count off for not specifying the rds or for writing both steps on a single line. Any base can be utilized.]
E. The Hoffmann Elimination
1.
[5 pts] Using resonance theory, develop a TS model for the generalized Hoffmann
elimination shown below and summarize as a DL/PC structure. Characterize this
structural model, specifying two distinct characteristics.
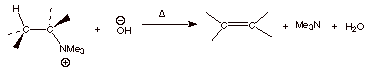
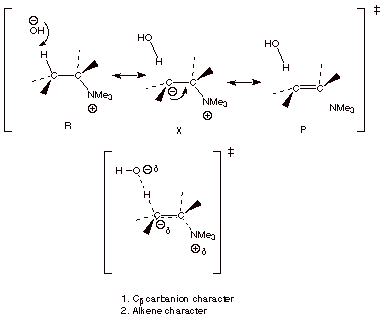
2.
[5 pts] Using this structural model, draw TS models for the formation of
1-butene and 2-butene in the Hoffmann elimination reaction shown below.
Indicate which TS state is favored and explain why.
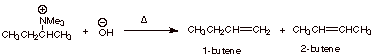
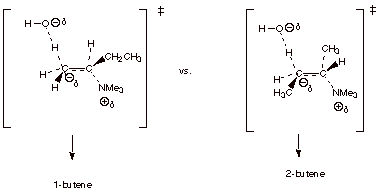
q
The TS leading to
1-butene is favored, because it has primary Cb carbanion character, while that leading to 2-butene has secondary
carbanion character.
q
Carbanions are
destabilized by alkyl groups: methyl more stable than primary than secondary
than tertiary.
3.
[1 pts] Of the two TS characters involved in E.1, which one is apparently the
dominant one? Explain your conclusion.
q
Evidently, the
carbanion character is dominant over alkene character, because alkene character
would have favored the more stable disubstituted alkene.
V.BENZYLIC RESONANCE/SYNTHESIS
A. Benzylic Resonance.
1.
[3 pts] Provide the resonance theoretical treatment of the benzyl carbocation,
summarize as a DL/PC, and characterize the ion (what character does it have and
where?).

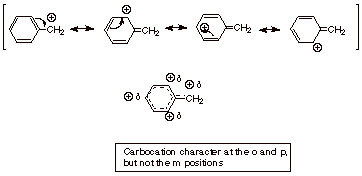
2.
[2 pts] What kind of nucleophilic substitution reactions does benzyl chloride
undergo? Explain.
![]()
q
It undergoes SN1
substitution reactions because it readily forms the relatively stable benzyl
cation.
q
It also undergoes SN2
reactions because it has an unhindered, primary carbon.
B. [2 pts each] Predict the structure of the main
product of the following reactions and briefly explain any selectivity elements involved.
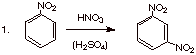
q
Nitro is an EWG, and
is meta directing.
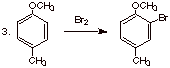
q
Both methoxy and
methyl are o,p directing EDG’s, but methoxy is the stronger EDG, so the
bromine goes ortho to methoxy, not ortho to methyl.
C. [2 pts] Sketch an efficient synthesis of the
following molecule, beginning with benzene. Explain any strategy involved in
the selection of this particular route.
![]()
![]()
q
The nitro group is meta
directing, so if it is installed first, the bromine will go into the desired
meta position. However, if bromination is carried out first, the o,p directing
nature of the halogen will cause the nitro group to go ortho and para to the
bromine, not the desired meta relationship between the two groups.