CH 610 A, 318M: Bauld
Third Exam; Fall, 2004
_____________________________________________
Name (Last, First)
CH 610A, 318M
Third Exam
Fall, 2004
Prof. N. L. Bauld
__________________________________________ Name (Last,
First)
Question |
Possible Points |
Page/Pts/Points Earned |
Score |
|
I. |
35 |
3/8/ 4/9/ 5/8/ 6/10 |
|
|
II. |
11 |
7/6// 8/5/ |
|
|
III. |
11 |
9/11/ |
|
|
IV. |
23 |
10/8/ 11/11/ 12/4 |
|
|
V. |
8 |
13/8 |
|
|
VI. |
12 |
14/12 |
|
|
Totals |
100 |
|
|
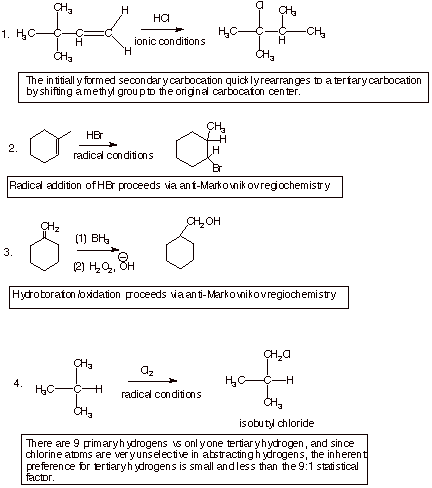
I. Acid Catalyzed Additions to Alkenes
A. Acid Catalyzed Addition of Methanol to Isobutene.
1. [5 pts] Write the detailed mechanism for the reaction shown below (the
acid-catalyzed hydration of isobutene) and specify the structure of the product
formed. In writing the mechanism, be sure to adhere to all of the conventions
of this course for writing rigorous reaction mechanisms.
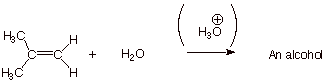
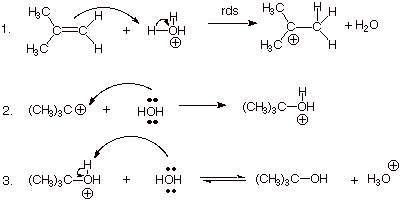
2.
[3 pts] Your mechanism should consist of
three steps, and you should have indicated one of them is rate-determining.
Explain in detail why this particular step is rate-determining, and also why
the other two are not rate-determining.
q
In the first step, two
bonds are broken (the pi bond and an O-H bond) and only one bond (a C-H bond)
is formed. This reaction is therefore strongly endothermic (and endergonic) and
is therefore likely to be the rds.
q
The second step involves
the formation of one bond and does not break any bonds. It is therefore highly
exothermic (and exergonic) and is not likely to be the rds.
q
The third step involves
breaking one O-H bond and forming one O-H bond. It is neither highly exergonic
nor endergonic, but proton transfers from oxygen to oxygen are typically
extremely fast.
3. [3 pts] For the rds of the above reaction, use resonance
theory to derive a TS model for this step. Be sure to use all of the course
conventions in employing resonance theory.
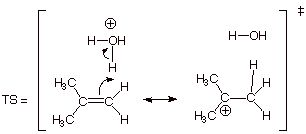
4. [3 pts] Summarize the above resonance treatment as a dotted
line/partial charge (DL/PC) structure and characterize this TS model as
accurately as possible (without employing the Hammond Principle).
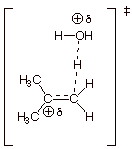
q
The TS has tertiary
carbocation character at the passive carbon.
5. [3 pts] State and apply
the Hammond Principle to this reaction step, explaining the energetic basis for your application.
q
Statement: The TS of a reaction step more closely resembles
the higher energy species of the reactant and product.
q
The reaction involves
the breaking of two bonds (the pi bond and an O-H bond) and the formation of
just one bond (a C-H bond). It is therefore highly endothermic (or endergonic),
so the product of the step is the higher energy partner.
q
The TS therefore more
closely resembles the carbocation, which is the product of the step. We can say
that the TS has extensive (or highly developed) carbocation character.
B. Relative Rates of
Hydration of Alkenes.
1. [6 pts] Using the TS model previously derived for the
rds of the acid-catalyzed hydration of isobutene, employ the Method of
Competing Transition States to
discuss the relative rates of addition of water to ethene, propene, and isobutene (you should display all three
TS models and specify their respective characters). Which reaction is the
fastest and why?
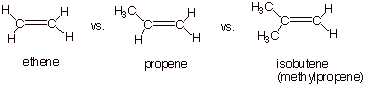
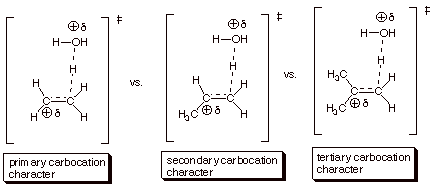
q
The reaction with
isobutene (the last one) is the fastest, because the TS has tertiary carbocation
character, which is much more favorable than secondary or primary carbocation
character.
2. [2 pts] Is the
ratio of the rates of hydration of propene vs. ethene expected to be large or
small? Give a specific, detailed reason for your answer.
q
Large, because the TS
has extensive carbocation character at the passive carbon. The more extensive
the carbocation character, the more sensitive the reaction is to the kind of
carbocation character which is present in the TS.
C. Stereochemistry of
Additions to Alkene Double Bonds.
1.
[3 pts] For the acid-catalyzed hydration
of 1,2-dimethylcyclohexene (see below), draw the structure(s) of the alcohol
product(s) formed.
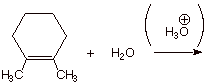
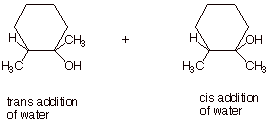
2.
[4 pts] Write out the detailed mechanism
for this reaction, and use this mechanism to assist you in explaining the
observed stereochemical result.
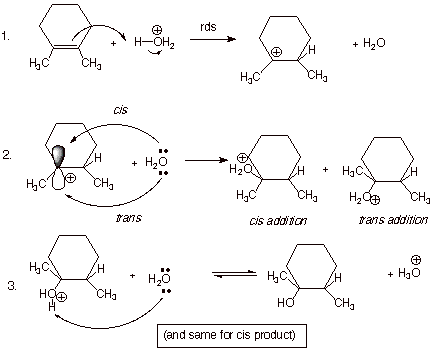
q
The intermediate
carbocation, formed in the first step, is sp2 hybridized at the
carbocation center, which is therefore planar. The resultant vacant 2p AO can
therefore react with the nucleophile (water) about equally from either the top
face (the one from which protonation occurred) or the bottom face. Therefore,
both the cis and trans products are formed (the trans is formed in slight
excess for steric reasons).
3. [1 pts] What
term is used to describe this kind of stereochemical result?
q
Non-stereospecific.
4.
[2 pts] What is the mechanistic symbol
for the reaction?
q
AdE2. It is
an electrophilic addition to the alkene (remember the viewpoint is that of the
organic molecule, which reacts with an electrophile, the hydronium ion, in the
rds.
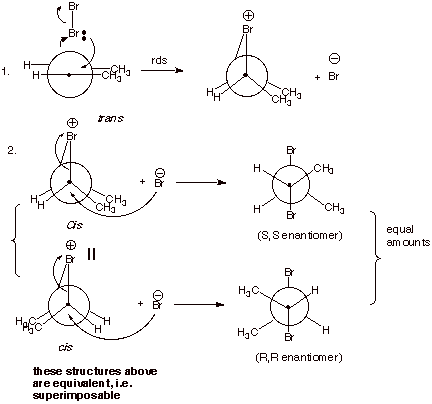
II. Bromination
A. Bromination of Alkenes
1. [4 pts] For the addition of bromine to cis and trans-2-butene,
draw Newman projection structures of all the product stereoisomers formed in
each case.
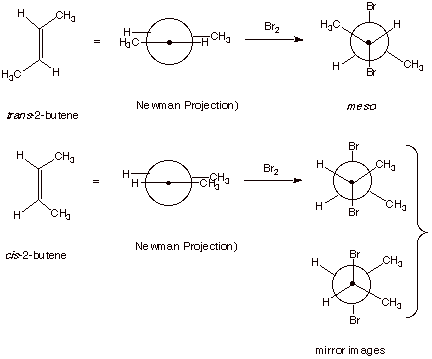
2. [2 pts] Which one of these products is the meso isomer? In
your Newman structure, what symmetry element does it possess?
q
Meso is formed from the trans 2-butene, as shown above.
q
It has a “center
of symmetry” or “point of symmetry”.
3. [2 pts] Are either of these product mixtures optically active?
Explain why or why not.
q
The meso isomer, formed
from trans-2-butene is achiral and
thus optically inactive.
q
The isomers formed from cis-2-butene are enantiomers, i.e., a racemic mixture is
formed (equal amounts of 2 mirror image isomers), so it is not optically active
either.
4. [3 pts] Provide a mechanistic explanation of why the
particular stereoisomers observed in each case are formed in these two
reactions. Employ Newman projection structures in your depictions.
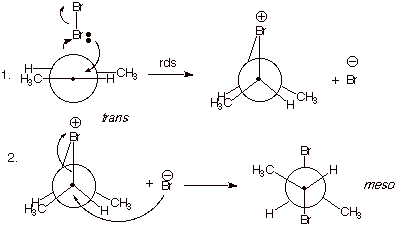
q
Bromine reacts to form
two bonds simultaneously, in forming the epibromonium ion. This is a concerted,
syn addition.
q
When the bromide ion
reacts with this ion, it reacts from the opposite face of the ion from the
bridging bromine.
q
In the case of
trans-2-butene, this forms only the meso isomer, but in the case of
cis-2-butene, the two enantiomers R,R and S,S-2,3-dibromobutane are formed (See
below).
III. Hydroboration/Oxidation
A. Hydroboration
1.
[3 pts] Draw the structure of the
two possible products in of the
reaction of 2-methyl-2-butene with borane and indicate which product is major.
![]()
![]()
q
The first product is the
major one.
2.
[5 pts] Provide a resonance
theoretical treatment of the TS for
the formation of the major product, summarize as a DL/PC model, and characterize the TS
carefully.
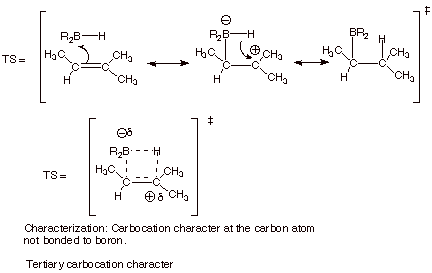
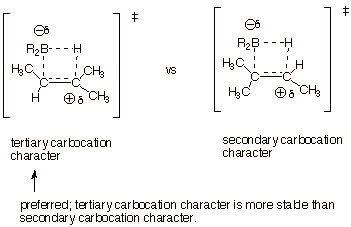
2. [3 pts] Use the Method of competing TS’s to rationalize the preferred orientation of the addition of borane to 2-methyl-2-butene. Your discussion should include TS models and characters for both modes of addition to 2-methyl-2-butene. Indicate which TS is preferred and explain why.
IV. Radical Stability; Radical Chain Reactions: Halogenation of Alkanes
A. Radical Stability
1. [4 pts] Arrange
the following radicals in order of decreasing stability (1= most stable) and
explain your reasoning.
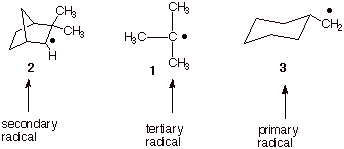
q
Tertiary radicals are
more stable than secondary than primary radicals.
B. Halogenation of Methane
1.
[3 pts] Thermodynamic Considerations
a. Provide an equation for approximating the standard enthalpy change (DHo) of a reaction and apply it to the chlorination of ethane, using the bond dissociation energies provided.
![]()
Bond
Dissociation Energies: D(H-Cl) = 103 kcal/mol; D(CH3CH2-Cl)
= 86 ;
D(CH3CH2-H) = 198 ; D(Cl-Cl) = 58.
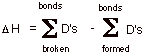
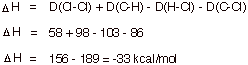
b. [1 pt] In an overall sense, what kind of reaction is this (the name of the reaction type)?
q Exothermic (accept also exergonic)
2.
The Reaction Mechanism
a.
[6 pts] Write the complete, detailed mechanism for the chlorination of ethane, specifying the three stages of the reaction.
C. Transition State Models
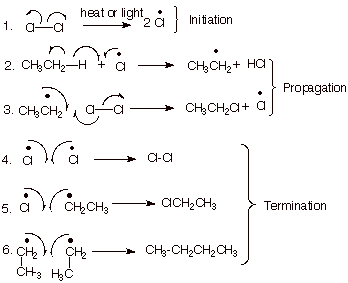
1. [5 pts] Provide a resonance theoretical description of the transition state of the reaction in which
chlorine atoms remove a hydrogen atom from ethane, summarize this as a dotted
line/partial radical character structure,
and characterize the TS model.
![]()
2. [4 pts] Extend
the DL/PRC structure from the
previous question to the case of bromination (abstraction of a hydrogen atom by
bromine atoms), and apply the Hammond Principle to refine your TS characterization. Use this refined
characterization to explain why bromination is highly selective for tertiary vs. primary C-H bonds.
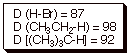
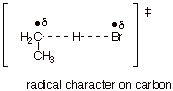
q The reaction with bromine atoms is rather strongly endothermic, because the CH bond (98 kcal/mol) which is being broken is stronger than the HBr bond, which is bing formed (only 87 kcal/mol). Therefore, the TS more strongly resembles the higher energy partner, which is the product, the ethyl radical.
q Consequently, the TS for bromination has extensive radical character.
q Since tertiary radical character is preferred over secondary or primary radical character, bromine atoms prefer to abstract tertiary hydrogens preferentially.
q
V. Grignard Formation
A. Mechanism of Grignard Formation.
1. [3 pts] Write the detailed
mechanism for the formation of the Grignard reagent shown below.
![]()
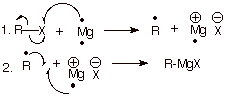
2. [2 pt] The mechanism is, of course, a radical mechanism, but
it differs in a basic respect from the other radical reactions we have studied.
In what general mechanistic respect does it differ from these others?
q It is a non-chain radical reaction.
3. [2 pts] Describe the bonding in the Grignard reagent,
including both the C-Mg and Mg-X bonds.
q The
C-Mg bond is polar covalent and
the Mg-X bond is largely ionic.
q The
carbon end of the C-Mg bond is the
negative end of the dipole.
4. [1 pts] What kind of character does a Grignard reagent have at
the carbon atom which is bound to magnesium?
q Nucleophilic character.
VI. Syntheses
A. Product Structures
1. [3 pts each = 12 pts] Draw the complete structure of the main product formed in each of the following reactions. Also, provide a brief explanation for any regioselectivity, stereospecificity (or lack thereof), or any other unusual aspect of the reaction. .