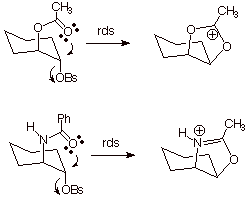
Anchimeric Assistance (Neighboring Group
Participation)
The
participation of neighboring groups in an SN reaction is revealed by
unique stereochemical results (retention in the substitution process) and also
(usually) by obvious rate enhancements in comparison to a model in which
neighboring group participation would be stereoelectronically impossible.
Consider the case of trans-2-iodocyclohexyl
brosylate (remember, brosylate is p-bromobenzenesulfonate, a very good leaving
group, better even than tosylate (why?)).
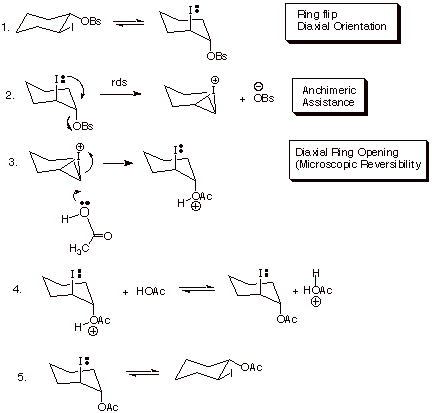
In
comparison to the corresponding cis
isomer, the rate of solvolysis in acetic acetic is more than a million fold
faster. This is because in the cis
isomer, the iodo substituent cannot approach from the backside of the carbon
which is undergoing substitution. Notice that a proper placement of leaving
group and entering nucleophile in an SN2 reaction requires a linear
(or near-linear relationship between the C-leaving group bond and the
C-nucleophile bond. This is feasible only when both the leaving group (here the
brosylate) and the participating nucleophile (here the iodo substituent) are
axial. This relationship is not available in the more stable diequatorial
conformer of the trans isomer, but
can be achieved readily by a ring flip to form the somewhat less stable diaxial
conformer. However, in the cis isomer, neither conformation available has the
appropriate diaxial relationship. The reaction therefore proceeds without
neighboring group participation, and as expected, via an SN1 reaction,
which gives a mixture of cis and trans product acetates. Incidentally, why would an SN1
mechanism be favored over an SN2 mechanism for this brosylate?
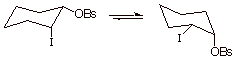
Iodo
substituents are powerful neighboring groups, but many other groups having
unshared electron pairs can do the same thing. Bromine produces a rate
enhancement of amost 103, while chlorine gives a much smaller rate
enhancement, along with analogous stereochemical results. Incidentally, an
acetate substituent is also a powerful neighboring group (rate enhancement ca. 103). Benzamido is extremely powerful as a
leaving group.