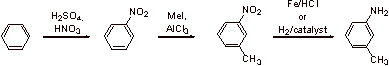CH 318N: Professor Nathan L. Bauld
Third Exam: KEY
Spring 2005
I. Electrophilic Aromatic Substitution
A1.
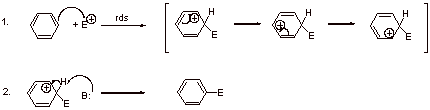
A2.
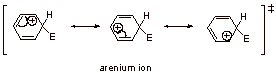
A3.
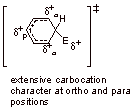
A4. This intermediate is not aromatic because the tetrahedral carbon to which the electrophile bonds, interrupts the cyclic conjugation.
A5. Elimination of the proton regenerates the aromaticity which would not be the case if a nucleophile added to the ortho or para position (would get non-aromatic cyclic diene).
A6. SE
B1.
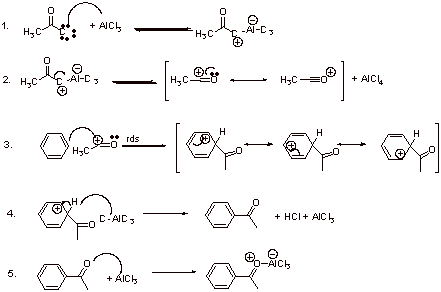
B2.
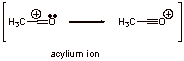
B3. No. Stoichiometric AlCl3 is required due to the last step in the mechanism. Irreversible Lewis acid – Lewis base complexation prevents AlCl3 to return to the catalytic cycle.
C1.
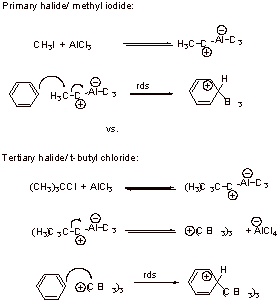
The primary halide undergoes concerted substitution whereas the tertiary (or secondary) halide undergoes stepwise substitution. Formation of carbocation is preferred with tertiary (and secondary) systems whereas primary carbocations are very unfavorable.
C2. Primary halides – SN2
Tertiary/secondary halides – SN1
II. Rates and Orientation
A1.
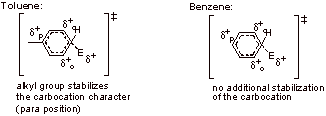
A2.
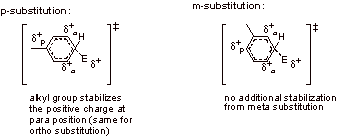
B1. EDG: ![]()
EWG:
![]()
B2.
![]()
The carbocation is resonance stabilized via the oxonium ion.
B3.
![]()
Both resonance forms that can be drawn for the nitro group have a positive charge on nitrogen, making it very electronegative and thus a powerful electron-withdrawing group.
C1.
The partial positve charge develops ortho and para to the electrophile in the EAS of benzene. When chlorobenzene undergoes EAS at either the ortho or para positions, the partial positive charge that forms ortho and para to the electrophile is on the carbon containing the chlorine. The rate is retarded because the chlorine destabilizes the forming positive charge via an inductive electron withdrawing effect.
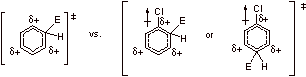
C2.
Para substitution of E+:

Para attack of the electrophile results in four possible resonance structures, including one where the chlorine can stabilize the positive charge by donating its lone pair electrons.
Meta substitution of E+:
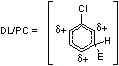
Meta attack of the electrophile results in only three possible resonance forms, as positive charge does not develop on the carbon adjacent to chlorine. The para transition state is favored due to this resonance effect.
III. Amines: Nomenclature, Properties, Synthesis
A1.
a. 1-butaneamine
b. 4-aminopentanal
A2. Primary amine: only one group other than hydrogen is attached to the nitrogen
B1. Alkyl groups are electron donating groups (EDG), which stabilize inductively the positive charge of the ammonium ion.
B2. Tertiary amines are sterically more hindered than primary or secondary amines and therefore additional stabilization of the ammonium ion by solvation is prevented.
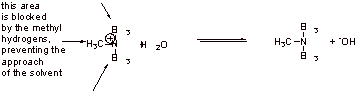
B3. The lone pair on the nitrogen in pyridine is sp2 hybridized (lower in energy) whereas the lone pair in aliphatic amines is sp3 hybridized. Pyridine lone pair is therefore more reluctant to abstract a proton (less basic) than a lone pair of an aliphatic amine. pKa of pyridine is ~ 5.25 whereas pKa of aliphatic amines is 9-10.
B4.
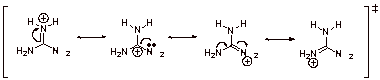
The ammonium ion of guanidine is strongly resonance stabilized (ammonium ion character and carbocation character; four resonance structures of which three are equivalent) and therefore it is very good proton abstractor.
B5. Aniline is less basic than simple 1o amines due strong resonance stabilization of the neutral compound:
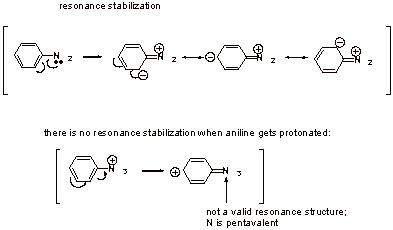
IV. Amines: Reactions
A1.
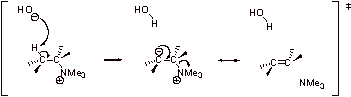
A2.
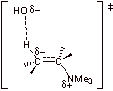
- Beta-C carbanion character
- Alkene character
A3. Products:
![]()
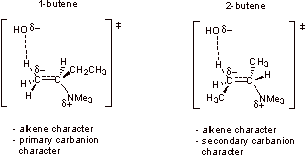
Recall that alkyl substitution stabilizes carbocations and destabilizes carbanions. 1-butene is the major product because there is primary carbanion character in the transition state. The transition state for 2-butene has secondary carbanion character, which is much less favorable. Thus 1-butene is the major product.
B1.
![]()
B2. The loss of N2 is very favorable and rate determining. Because N2 is a gas, it is removed from the reaction, making this step irreversible and addition of the nucleophile proceeds via an SN1 mechanism.
B3.
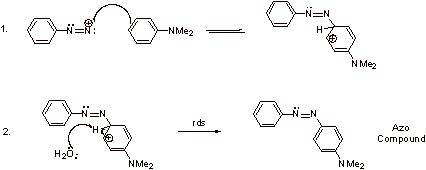
V. Synthesis
A1.
![]()
A2.
![]()
Methyl and methoxy are both ortho, para directing groups. The methoxy group is more strongly electron donating than methyl, as it can stabilize the forming positive charge by resonance. The major product is thus dictated by the directing effect of the methoxy group and the bromine will be ortho to the methoxy.
A3.
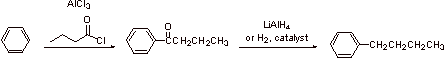
B1.
![]()
B2.
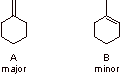
A is the major product because the transition state leading to A has primary carbanion character and the transition state leading to B has secondary carbanion character.
B3.