CH 318N: Professor Nathan L. Bauld
Final Exam: KEY
Spring 2005
I. Conjugated Dienes
A1.
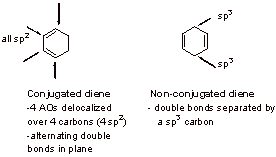
A2.
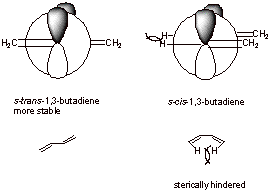
A3.
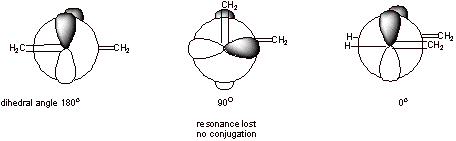
The bond between carbons 2 and 3 has some pi character, so it is not ‘pure’ single bond. Rotation around this bond has to go through conformation where the dihedral angle is 90o and is not favorable. 8 kcal/mol is the barrier for this rotation to happen.
B1.
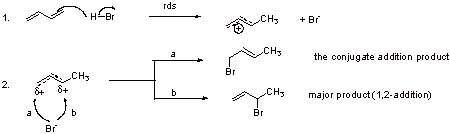
B2.
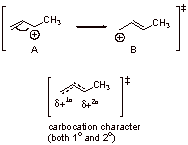
The structure A is lower in energy than B (secondary carbocation character vs. primary carbocation character; +δ2o > +δ1o) and therefore bromide reacts faster with A.
B3.
The major product at room temperature is the conjugate addition product which is the more stable product. This product has a disubstituted double bond whereas the 1,2-addition product has monosubstituted double bond.
B4.
At -78 oC, kinetic control results in the observed product. Kinetic control means that the reaction is controlled by the competing transition states and which one occurs faster.
At room temperature the reaction is thermodynamically controlled which means that the product is controlled by the stabilities of the final products.
C1.
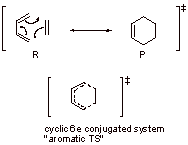
Diels-Alder reaction is very facile since the transition state is highly favorable aromatic transition state.
C2.
These results suggest syn stereospecificity and a concerted mechanism. Starting from cis-alkene results in ‘cis’ product and likewise the trans-alkene gives ‘trans product. If the reaction was stepwise where i.e. diradical intermediate would exist, we would observe mixtures of products.
C3.
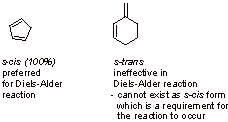
C4.
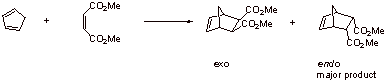
II. Polymers
A1.
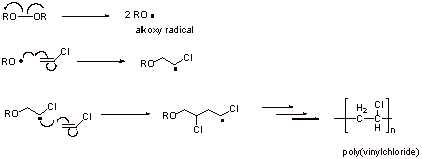
A2.
a. The first structure is preferred since the radical is stabilized by chlorine substituent. The second structure has less favorable primary radical character.
b. Regioselectivity
c. Head-to-tail regiochemistry
A3. a. Not the linear structure but a branched:
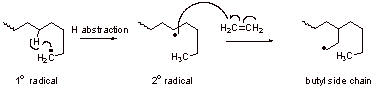
b. The branched polymer has lower melting point and is of lower density than the linear polymer.
B1. a. syndiotactic
b. isotactic
c. stereorandom or atactic
C1.
- Rubber has Z-double bonds which are less stable than E-double bonds.
- Competition of 1,2- and 1,4-addition
C2.
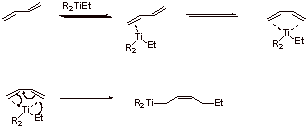
Coordination of the Z-N catalyst to the diene provides s-trans type complex which rotates around the C-C bond to give even more stable coordination complex (both pi bonds of the diene are able to complex with Ti). When the addition occurs in the conjugate manner, the C=C is generated in the cis configuration. The 1,4 addition mode may be favored because of the six-membered ring TS.
D1.
D2.
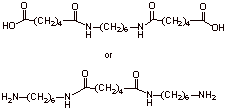
D3.
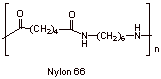
D4.
Chain-growth polymerization proceeds fast to give the polymer from the monomers without intermediates.
Step-growth polymerization involves detectable lower oligomer intermediates; dimers, trimers, etc. which then can combine to give a polymer. Step-growth polymerization is slower than chain-growth polymerization.
III. Carbohydrates
A1.
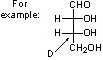
This structure corresponds to the family name as there are four carbons in the main chain and it is an aldehyde.
A2.
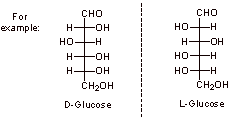
A3.
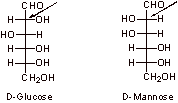
D-Glucose and D-mannose are very closely related structurally as they only differ in the stereochemistry at C2 (indicated above). Mechanistically, one can be converted to the other via the formation of the enol or enolate, followed by protonation, which could occur from either face of the molecule (interconversion via the enolate is shown below).
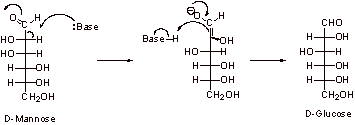
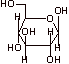
B. 1. a. Beta-D-glucopyranose
b.
![]()
c. Hemi-acetal functional group at carbon 1
d. Beta-D-mannose is less stable than structure A because it has an axial hydroxyl group at C2, where structure A does not.
![]()
C. 1.
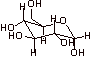
If the group (OH or H) is below the ring on the chair, it points down on the Haworth structure.
D. 1.
 B
B
alpha-D-glucopyranose
2. The change in optical rotation is due to mutarotation, or equilibration of the alpha and beta forms (B and A) via opening up to the aldehyde. The change in optical rotation is due to the fact that the alpha anomer is present in 36% in the final equilibrated mixture and the beta anomer is present in 64%. The free aldehyde comprises less than 1% of the mixture.
3. The specific rotation will not change. Deprotonation of the hydroxyl group at C1 is required for equilibration to occur under basic conditions. The ketal can only open and equilibrate under acidic conditions.
4. Methyl beta-D-glucopyranoside
5.
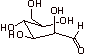
E. 1.
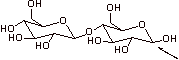
cellobiose
2. Yes, because it can interconvert with the aldehyde via the free anomeric carbon (indicated by the arrow on the above structure). The aldehyde can be reduced, which is why this is called a reducing sugar.
3.
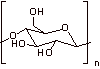
cellulose
4. No, this is not a reducing sugar. There is no anomeric carbon with a free hydroxyl that can open to the aldehyde.
IV. Lipids
A. 1.
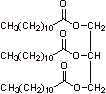
2.
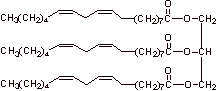
B. 1.
![]()
Stearic acid
2.
![]()
Linoleic acid
C. 1.
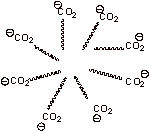
(1) The molecules cannot completely dissolve in water because the nonpolar fatty acid portion of the molecule is insoluble.
(2) The polar CO2- portion of the molecule is soluble in water so the molecules
couldn’t completely precipitate out.
Because of (1) and (2) the fatty acids minimize interaction of the greasy portion of the molecule by placing it on the inside of this micelle. The polar portion then interacts with the water while the nonpolar portion interacts with itself. The micelle then exists as a suspension in water.
2. No, the length of the fatty acid chain portion of the molecule is not enough to make the Van der Waals attractions between the ‘tails’ enough to afford a stable interior for the micelle.
3. Nonpolar substances, like grease, will collect in the interior of the micelle while the polar portion allows this grease to dissolve in water.
- The advantage of this salt as a cleansing agent is that the SO3- group is compatible with hard water (which contains Ca2+ and Mg2+) while soap is not. With soap, the Ca2+ and Mg2+ salts precipitate out as “scum,” whereas in detergents they are soluble.
D. 1.
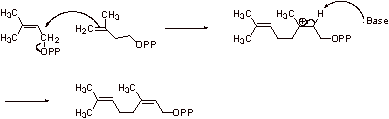
2. The pyrophosphate is a good leaving group.
3.
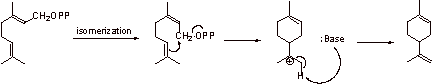
4.
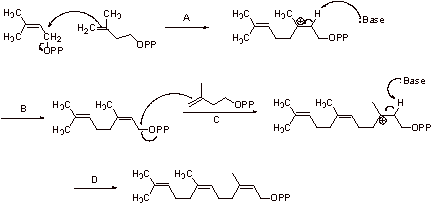
Step A: The allylic pyrophosphate is displaced to give a tertiary carbocation.
Step B: Elimination gives another allylic pyrophosphate.
Step C: The double bond attacks, displacing this allylic pyrophosphate and forming a
tertiary carbocation.
Step D: Elimination gives the triene.
V. Amino Acids, Peptides, and Proteins
1.
![]()
2. Zwitterion
3.
![]()
4. Arginine is the most basic, because the protonated form has four resonance structures, three of which are equivalent:
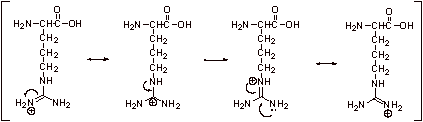
5. Compounds with higher pKa’s are weaker acids, and thus stronger bases. Because the pI is the point halfway between the cationic form and zwitterions, the pI will also follow this trend, and the most basic amino acid will have the pKa of 10.76.
6. Aspartic acid is the more acidic amino acid because the acidic proton is one carbon closer to the electron withdrawing carboxylic acid group than in glutamic acid.
B. 1. a.
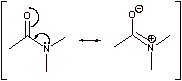
Recall that amides are highly resonance stabilized, making them sp2 hybridized. The p orbital on N is aligned with the C-O p orbital in order for the lone pair to engage in resonance as shown above:
Amines are tetrahedral and sp3 hybridized as the nitrogen is not involved in resonance that would give it sp2 character.
b. In a simple amine or peptide bond 6 atoms are required to be planar. This is because the amine must be coplanar with the carbonyl to allow for resonance (above). Because the nitrogen and carbon indicated are sp2, they have trigonal geometry placing all of the atoms that they are attached to in the same plane. Thus all of the atoms shown below must be in the same plane.
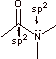
c. The two conformations are the trans and cis conformations. Due to the extensive double bond character between the N and carbonyl carbon, their interrconversion is relatively difficult. The trans isomer is somewhat more stable due to steric interaction between the R groups (shown below):

d. The amide-type oxygen is a better hydrogen bond acceptor, as there is more negative charge on the oxygen due to the resonance with the nitrogen. Nitrogen is less electronegative than oxygen, and can stabilize the positive charge in the resonance form better than the oxygen in the ester.
C1.
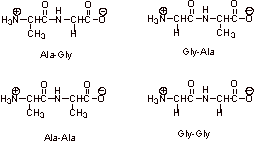
C2.
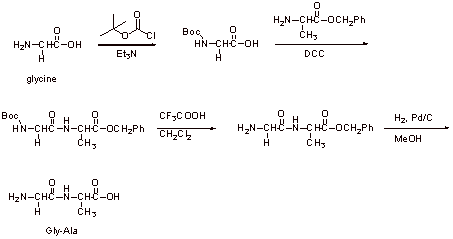
It is important to protect the amino group of the glycine to prevent its coupling to the alanine carboxylic acid site and the protection of the alanine carboxylic acid as the benzyl ester prevents its coupling to itself or to the glycine amino site. There could be 4 possible products otherwise (see previous question).
D.
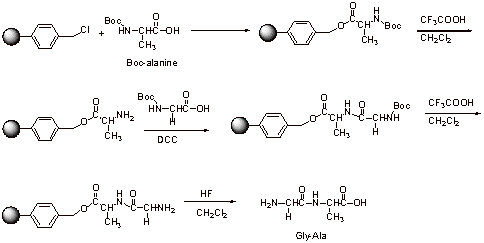
Extra Credit
1.
![]()
2. Epoxide Formation