CH 318N: Professor Nathan L. Bauld
Second Exam: KEY
Spring 2005
I. Enol/Enolate Formation, Structure, and Reactivity
A1.
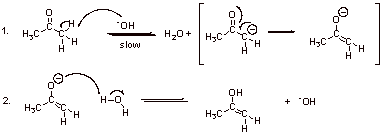
A2.
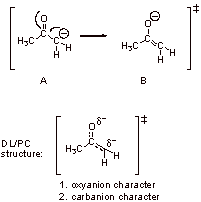
A3. Structure A is favored because of the carbonyl group (vs. alkene double bond in B).
Structure B is favored because of the oxyanion character (vs. carbanion character in A).
Therefore A and B are approximately equal in energy.
A4. pKa 19 corresponds to Ka = 10-19 and pKa 16 corresponds to K = 10-16. Thus, the equilibrium constant for this reaction is:
![]() which means that the
equilibrium lies to the left.
which means that the
equilibrium lies to the left.
A5.
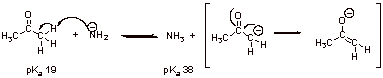
In this case the equilibrium constant for the reaction is:
![]() which means that the equilibrium lies to the right.
which means that the equilibrium lies to the right.
B1.
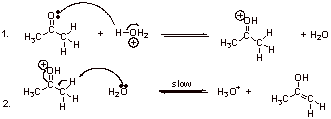
B2.
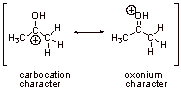
C1.
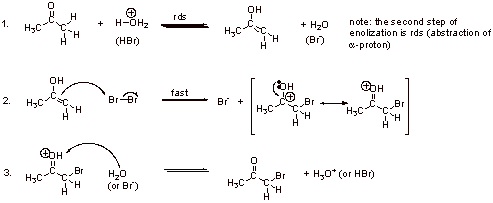
C3. The electron rich enol double bond is much more reactive towards electrophiles (Br2) than a simple double bond (electron poor). This is because of the resonance stabilization of the enol (negative charge on the carbon and positive on the oxygen makes the enol even more nucleophilic.
II. Aldol and Claisen Reactions
A1.
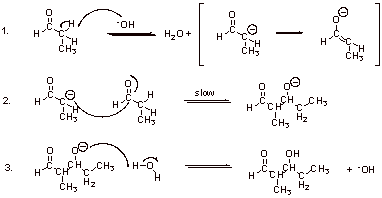
A2.
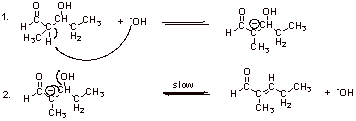
Elimination is unusual since –OH is a poor leaving group. However, at higher temperatures intramolecular OH elimination occurs often.
A3. Aldol condensation is important for ketones since the aldol addition is slower for ketones than for aldehydes. The equilibrium constant is less favorable for ketones to undergo an aldol reaction. The aldol condensation provides a more stable a,b-unsaturated ketone that cannot further react in a crossed aldol condensation manner like an a,b-unsaturated aldehyde could.
B1.
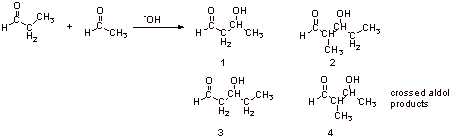
Four possible products, therefore not very useful reaction.
B2.
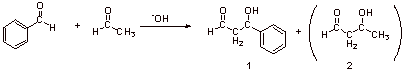
One major product 1. Benzaldehyde cannot enolize since it doesn’t have a-protons.
B3. Not a useful reaction since both starting materials are lacking a-protons that are needed for enolization; No aldol products.
C1.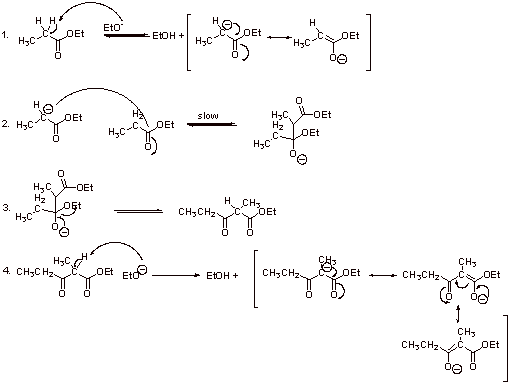
C2. The first enolization is uphill; pKa of the ester is about 21 and the pKa of the conjugate acid of EtO- 16. Therefore, the equilibrium constant for the first step is:
![]()
Reaction goes to completion because the pKa of the b-ketoester (a-proton, step 4) is about 10 and the pKa of the conjugate acid of EtO- is about 16. Therefore, the the equilibrium constant for this exergonic neutralization reaction is:
![]()
The overall thermodynamic driving force is
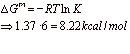
C3. Steps 1-3 convert one ester functionality to a ketone functionality, thus forfeiting the ester resonance, which would be unfavorable without the exergonic driving force discussed above.
III. Direct and Indirect Alkylation of Ketones and Esters
A1.
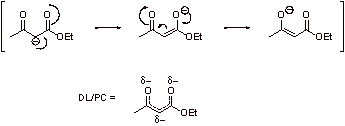
A2.
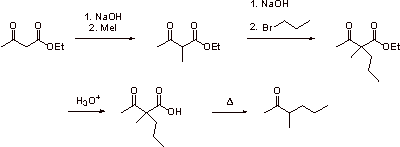
A3. The ketone cannot be synthesized by an analogous route. Only two alkyl groups can be introduced onto the enolate carbon of ethyl acetoacetate. Decarboxylation forms an enolate, which tautomerizes to a ketone. Through ketonization, a proton is introduced. Another alkyl group would have to be introduced to make the ketone, thus the ketone is not accessible via an analogous method.
A4.

B.
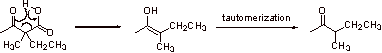
A concerted mechanism is involved in the above reaction.
C.
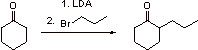
IV. Aromaticity
A1. Kekulé structure shows only one resonance form and localized double bonds as if there were alternating short and long bonds in the benzene ring.
Kekulé structure suggests energy of alkene structure that is not as stable as aromatic structure.
A2. The number of pi electrons is 4n + 2 and the system needs to be cyclic and fully conjugated.
A3. 36 kcal/mol
B.
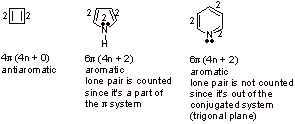
C.
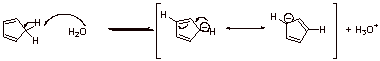
D.
![]()
V. Phenols
A1. pKa of phenol » 10
pKa of ethanol » 16
A2.
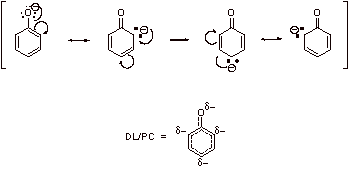
Highly resonance stabilized anion
A3.
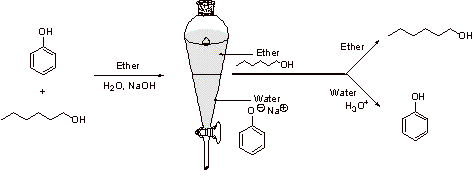
Because phenol is more acidic than hexanol, phenol can be selectively deprotonated with hydroxide. The hexanol will remain protonated in the presence of NaOH and when water and ether are added, it will go into the ether layer. Phenoxide will go into the aqueous layer. The layers can be separated, then the aqueous layer can be acidified to protonate phenoxide to give phenol.
A4.
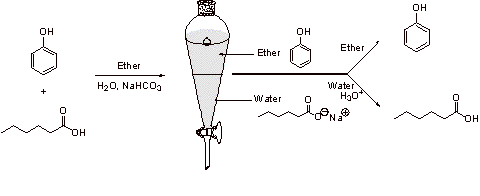
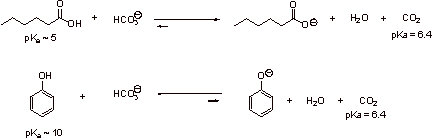
Because carboxylic acids are stronger acids than phenols, carboxylic acids can be deprotonated with sodium bicarbonate (NaHCO3) to a greater extent than phenol. Phenol is not extensively neutralized, thus the phenol will go into the organic ether layer. The sodium salt generated by the deprotonation of the carboxylic acid will go into the aqueous layer (Note the direction that the equilibrium is shifted in the reactions below.):
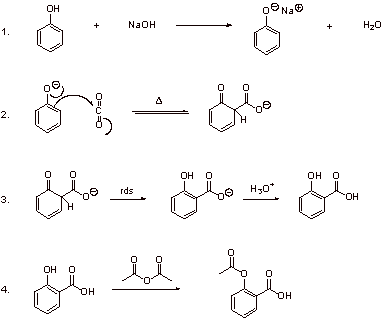
A5. Commercial synthesis of aspirin:
A6. The enol form of phenol is more stable, as the double bond of the enol is involved in part of an aromatic system. The keto form is not aromatic, thus not as stable.
![]()
VI. Nuclear Magnetic Resonance Spectroscopy
1.
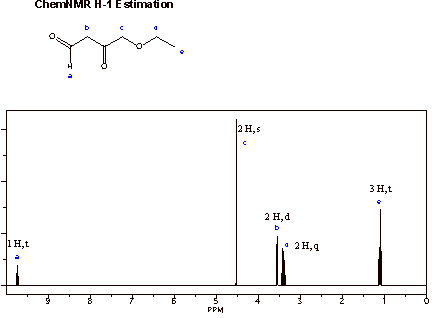
Expansion of the region from 5-0 ppm:
s = singlet
d = doublet
t = triplet
q = quartet