UNIT 7:CATION RADICALS
Cation radicals are species
which are formed from neutral molecules by the removal (ionization) of a single
electron. The resulting species therefore has a unit of positive charge and a
unit of spin density (Scheme 1). The HOMO of the neutral species becomes the
SOMO of the cation radical. Remember that the SOMO completely controls the spin density distribution. Also, in
alternant hydrocarbon (nonpolar) systems, it also controls the charge density,
i.e., spin and charge travel together. This means that wherever the spin is
large the charge is also large. Consequently, radical reactivity and ionic
reactivity tend to occur at the same atoms.
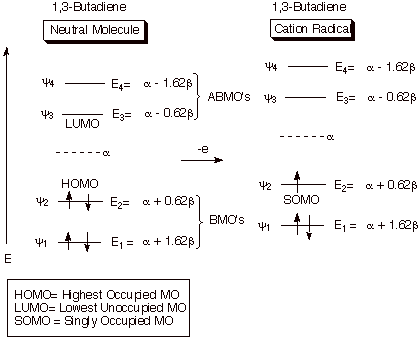
Scheme 1. Illustrating the formation of a cation radical by the
removal of an electron from the HOMO of a neutral molecule.
Recall,
also, that the HOMO and the LUMO of 1,3-butadiene are paired, since it is an
alternant system (has no odd-membered rings). Consequently, the spin and charge
distribution in the cation radical are the same as in the anion radical. From
the coefficients of the HOMO and LUMO of 1,3-butadiene (given earlier), you
should be able to calculate the spin and charge distribution of both the anion
and cation radical, and to verify that they are the heaviest on the terminal carbons
(C1 and C4).
There
is one other thing I’d like for you to notice about both the anion
radical and the cation radical, viz., that the bond order between C2-C3 is
increased in both relative to the neutral molecule, making it much more
resistant to rotation, and therefore making it more difficult to interconvert
the s-cis and s-trans conformations (Scheme 2). This is especially easy to see
In the anion radical, because the extra electron is going into a LUMO which is
bonding (has coefficients of the same sign) between C2-C3. But in a similar
way, in the cation radical an electron is being removed from the HOMO of
butadiene, and in this MO the interaction between C2-C3 is antibonding. So an
antibonding interaction is being removed, resulting in a larger net bonding
between C2-C3.
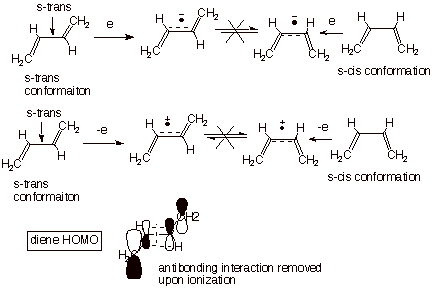
Scheme 2. Upon
either cation radical formatioin or anion radical formation, the C2-C3 bond
order is sharply increased, resulting in s-cis and s-trans conformations of the
ion radicals which are not readily interconverted, in contrast to the easy
conversion in the neutral diene.
Stable Cation Radicals
Like
neutral radicals and anion radicals, cation radicals can be isolably stable if
there is sufficient provision for extensive conjugative stabilization and also
steric effects which hinder dimerization. An excellent, and useful, example of a stable cation
radical is the tris(4-bromophenyl)aminium hexachloroantimonate salt, a
shelf-stable, commercially available, deep blue salt (Scheme 2). This salt is
prepared by the oxidation (using antimony pentachloride) of
tris(4-bromophenyl)amine. The removal of an unshared electron pair from
nitrogen generates a cation radical moiety, which is delocalized over all three
phenyl rings, primarily at the ortho and para positions of the ring. The bromo
substituent in the para position is necessary in order to prevent the cation
radical from dimerization at the para position.
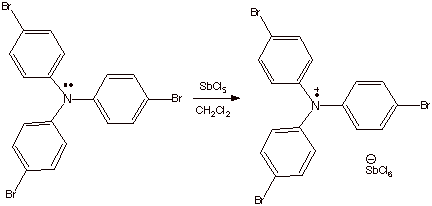
Scheme 3. A Stable Cation Radical Salt:
Tris(4-bromophenyl)aminium hexachloroantimonate
Since
a bromine substituent is an electron withdrawing group (an EWG), it destabilizes positive charge (which is located in part at the para position), This cation radical is therefore less
thermodynamically stable than the corresponding unsubstituted one would be,
even though it is more highly stabilized (sterically) against dimerization. It
is especially useful for acting as a rather reactive one electron oxidant,
i.e., for removing an electron from certain substrates in order to transfer the
cation radical moiety to the substrate. This is illustrated in Scheme 4, where
the cation radical of 1,3-cyclohexadiene is generated using this aminium salt,
and it undergoes an extremely fast Diels-Alder reaction with a molecule of the
neutral diene. This represents a powerful way for catalyzing the Diels-Alder
reaction, and it works best with relatively easily ionizable substrates which
would not undergo the normal, thermal Diels-Alder reaction very efficiently.
Cation Radical Cyclobutanation
Many relatively easily ionizable alkenes can undergo
efficient cyclodimerization to yield cyclobutanes (cyclobutanation) via a
similar cation radical mechanism. See if you can write out the cation radical
chain mechanism for the cyclobutadimerization of trans-anethole (Scheme 5). Recall that thermal combination
of two alkene moieties to give a cyclobutane is extremely difficult.
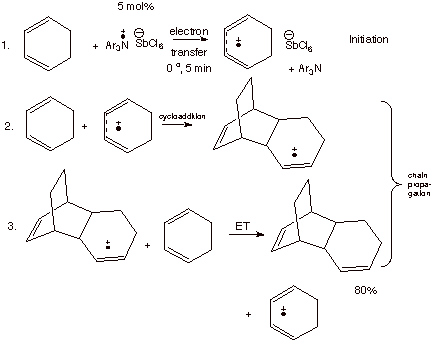
Scheme 4. The Cation Radical Diels-Alder Reaction
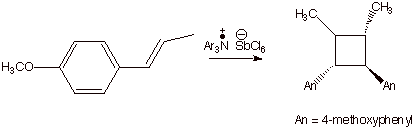
Scheme 5. Cation Radical Chain Cyclobutadimerization
Interestingly,
anion radical chain reactions can accomplish analogous cyclobutanations. In
this case, the substrate must be readily able to accept an electron, i.e., have
not too negative a reduction potential. This is provided by conjugation in
combination with strongly electron withdrawing groups (Scheme 6). See if you
can draw out an an anion radical chain mechanism for cyclobutanation which is
analogous to that for cation radical chain cyclobutanation.
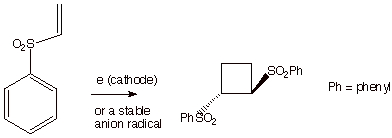
Scheme 7. Anion Radical Chain Cyclobutanation