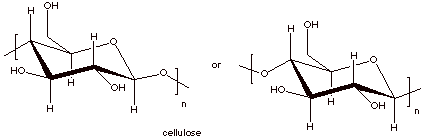Carbohydrates
The
name carbohydrates applies to certain naturally occurring compounds which are
basically polyhydroxy aldehydes or ketones. Of course, not all polyhydroxy aldehydes or ketones are naturally
occurring and thus would not be considered to be carbohydrates. Familiar
examples of carbohydrates (which are also called sugars and saccharides) are sucrose, glucose, ribose, starch, and cellulose.
Before we discuss the structures and chemical reactions of carbohydrates, let’s consider some simple, prototype molecules which are structurally related to carbohydrates.
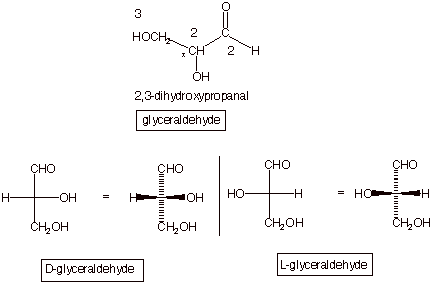
Glyceraldehyde.
Glyceraldehyde
is a common name for the substance the IUPAC name of which is
2,3-dihydroxypropanal (see below). It has only two hydroxyl groups, so it is
merely a prototype of the true carbohydrates. We note that C2 of
glyceraldehydes has four different substituents, an H, an OH, a CH2OH,
and a CHO substituent. That carbon is therefore a stereocenter. Since there is only one stereocenter, this means that the molecule is chiral; there are two enantiomers. We could classify them according to the R,S
nomenclature that we previously learned, but in sugar chemistry the
designations are D and L and the method used to derive the appropriate
designation is unrelated to that used to derive the classification of R or S.
q
Please recall the
conventions associated with Fischer structures, especially that vertical groups
project directly away from the observer (behind the plane of the paper) and
horizontal groups project outward toward the observer.
q
According to the
conventions originated by Nobel Laureate Emil Fischer, in writing carbohydrate
structures, the aldehyde function is represented as the upward vertical group
and the terminal hydroxymethylene group as the downward vertical group. When
this convention is followed the hydroxy and hydrogen atoms of the various
stereocenters (only one in this case) will be horizontally projecting groups.
One of the enantiomers will have the OH projecting to the right and the other
enantiomer to the left. The one which has the OH group projecting to the right
is called the D enantiomer.
q
Fischer structures are
especially useful, as we shall see for displaying the stereochemistry at
multiple stereocenters simultaneously and in a manner which facilitates
discovering any planes of symmetry which may identify meso compounds.
Erythrose and Threose.
It should be noted that the characteristic suffix of carbohydrates is –ose. We want now to look at the next higher members of the series which begins with glyceraldehydes, namely erythrose and threose.
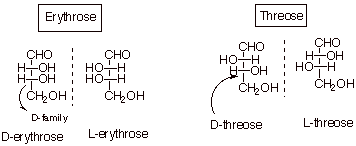
q
Both erythrose and
threose are 2,3,4-trihydroxybutanals.
q
Both C2 and C3 are
stereocenters, and they are non-equivalent, so there are 2n = 4
stereoisomrs; i.e., 2 diastereoisomeric pairs of enantiomers.
q
In the conventional
orientation, erythose is the diastereoisomer which has both OH’s oriented
in the same direction (both to the right or both to the left). Threose is the
diastereoisomer which has the OH groups oriented in opposite directions (one to
the right and one to the left).
q
IMPORTANT: The enantiomer is designated as D which has the OH at
C3 oriented to the right. In general, the farthest stereocenter from the
carbonyl function serves as the basis for the D or L designation, and the
molecule which has the OH oriented to the right at this remote stereocenter is
designated as the D enantiomer.
Subclassifications
of Carbohydrates.
q
Carbohydrates which have
an aldehyde function, as illustrated above, are called aldoses, while those which have keto functions are called ketoses.
q
Carbohydrates are
further classified according to the number of carbon atoms in the main chain,
e.g., erythrose is an aldotetrose. A carbohydrate which has 5 carbon atoms in
the chain is called a pentose and glucose and others which have 6 carbon atoms
in the chain are called hexoses. More specifically, glucose is an aldohexose.
q
We shall see that
all naturally occurring carbohydrates are of the D family. The L enantiomers
are not found in nature, but can be prepared in the laboratory.
Aldopentoses
Aldopentoses
are of the family 2,3,4,5-tetrahydroxypentanal. Since there are 3
stereocenters, there are a total of eight stereoisomers; four diastereoisomeric
pairs of enantiomers. Below are given the structures of D-ribose and D-arabinose,
two especially important carbohydrates.
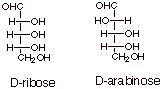
q
Note that D-ribose has
all three hydroxyl groups to the right. Ribose is important because of its
presence in RNA. Deoxyribose, which has lost its C2 OH (it is replaced by an
H), is correspondingly important in DNA.
q
Arabinose is of note in
the present connection mainly because its structure is so closely related to
that of D-glucose, as we shall see.
D-Glucose and
Other Aldohexoses and Ketohexoses.
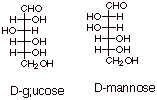
Glucose belongs to the family 2,3,4,5,6-pentahydroxyhexanal. The specific stereoisomer which we call D-glucose is shown below. The structure of D-mannose is also shown for reasons which will become apparent. Note that D-mannose differs from D-glucose only by the configuration at C2.
q
Note that glucose has 4
stereocenters, so that there are 16 stereoisomers, consisting of 8 different
diastereoisomeric pairs. Of course only the D family of diastereoisomers is
naturally occurring.
q
Just for practice,
L-glucose is shown below as the mirror image of D-glucose. Note that the
right/left configuration of every H/OH pair is inverted.
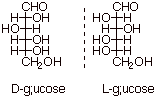
Glucose and Arabinose
Glucose is an aldohexose, while arabinose is an
aldopentose. However, the structures are somewhat related in that the
stereochemical configurations of the bottom 3 (3 of the 4) stereocenters of
D-glucose is exactly that of the three stereocenters of D-arabinose. Thus if
one knows the Fischer structure for glucose (which one should know!!), one
would also be able to write the structure of arabinose.
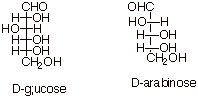
Glucose and Mannose
In a similar way, the bottom three stereocenters of
D-glucose and D-mannose are identical, so that knowing the structure of
D-glucose one can easily derive the structure of D-mannose.
Glucose and Fructose
D-Fructose (fruit sugar) is found in a chemically bound
form in table sugar (sucrose). It is a ketohexose which again has the same
configuration as D-glucose at the bottom (and only) three stereocenters.
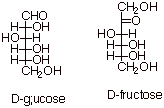
Cyclic Structures
We are award that aldehydes react with
alcohols to give hemiacetals, and that this is especially favorable when the
aldehyde function and the hydroxyl function are both present in the same
molecule. In this situation, cyclic hemiacetals are formed. D-Glucose, and
other sugars, fulfill these requirements and typically exist predominantly in
the cyclic hemiacetal form.
q
It is the OH
at C5 which preferentially adds to the aldehyde function, because this results
in a relatively strain-free six-membered ring. However, cyclization can
sometimes occur using the OH group at C4, which provides a 5-membered ring.
q
The
six-membered ring hemiacetal is called a –pyranose in general. In the
case of D-Glucose we have D-glucopyranose. The corresponding 5-membered ring
hemiacetal is called D-glucofuranose.
Naturally occurring D-glucose exists only in the pyranose form, but in solution
in the laboratory, much of the furanose form is also present.
q
It is not
important to note that the acetal carbon has four different substituents, and
thus is a new stereocenter. The molecule now has 5 stereocenters, affording 32
possible stereoisomers, Again, however, only the D enaniomers are present.
q
This means
that there are now twice as many diastereostereoisomers (16) as there were
before in the acylic form (8).
q
The two
diastereoisomers of D-glucose are shown below. It is very important to note
that the b-diastereoisomer has every substituent
equatorial on the chair cyclohexane ring. The a-stereoisomer has one axial OH, so it is somewhat less stable than
the b diastereoisomer.
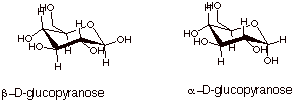
q
Diastereoisomers which differ
only in their configurations at C1 (the hemiacetal carbon) are sometimes called
anomers. They are readily
interconverted by equilibrating with the open chain, free aldehyde form.
q
We would like to note
here that knowing the conformational structure of glucopyranose, we could
immediately write the structure of D-mannopyranose, since it differs only by
the configuration at C2. Note that even the b diastereoisomer of
D-mannopyranose has one OH group equatorial and thus it is less
thermodynamically stable than b-D-glucopyranose.
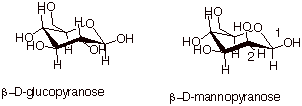
q
So glucopyranose is the
most stable of all of the pyranose structures.
Interconvertibility of Glucose and Mannose
q
Note that, in their free
aldehyde form, glucose and mannose differ only in their configurations at C2,
which is an alpha type carbon (alpha to a carbonyl group), which therefore
possesses acidic alpha type protons, which are easily removed to form the enol
(in acidic solutions) or enolate
(in alkaline solutions). Once the enol or enolate is formed, the alpha carbon
is planar (sp2), and when it is protonated, it can be protonated
from either face of the double bond.
Interconvertibility of The Glucose Anomers
q
The alpha and beta
anomers of glucose (or any of the other stereoisomers of glucose) are
especially readily interconverted in the presence of even traces of acid or
base, producing an equilibrium mixture of the two anomers.
q
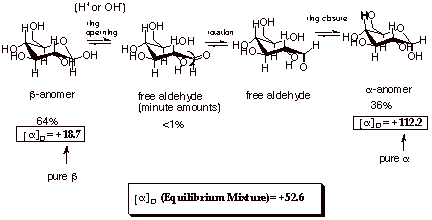
This is accomplished via
the free aldehyde form, which we know is in equilibrium with the cyclic acetal
form whenever even small amounts of acid or base are present (See the scheme
below).
q
If we start with the
pure beta anomer, it will rapidly rearrange to the equilibrium mixture of
64:36. Likewise, if we start with the pure alpha anomer, it will gradually
rearrange to the same equilibrium mixture.
q
Since the alpha and beta
anomers are diastereoisomers, they have different optical rotations, as shown
above. The alpha anomer is more strongly dextrorotatory than the beta anomer.
q
As the isomerization
takes place, the optical rotation gradually changes from that of the pure
anomer to that of the equilibrium mixture, which is intermediate between the
two. This phenomenon, often observed in sugars, is called mutarotation.
q
Note that mutarotation
in basic or neutral solution can only
occur when we have the hemiacetal function (or a hemiketal function), since the
OH group of the acetal function is necessary to equilibrating it with the
aldehyde form. In particular, if we have an acetal function, it is stable in basic solution and will not
permit mutarotation.
Glycoside
Formation.
Glycosides are acetals which correspond to the replacement of the
hemiacetal hydroxyl group of a sugar being replaced by an –OR group,
which R is any organic group.
q
A glycoside of the sugar
glucose is called a glucoside. A glycoside of mannose, would be called a
mannoside.
q
We are well aware that
hemiacetals can be converted to acetals in the presence of an alcohol, but only
in acidic solution.
q
The equation below
illustrates the conversioni of b-D-glucopyranose to methyl b-D-glucopyranoside in the presence of methanol. The R group in this
case is CH3.
q
Note that the typical
suffix for a glycoside is “-oside”.
q
The mechanism for acetal
formation has been studied previously this semester and is not repeated here
[protonate the OH; lose water as the leaving group; the carbocation then reacts
with methanol to give the acetal; note: both alpha and beta anomers will be
formed, because the intermediate carbocation can react with methanol from
either face.
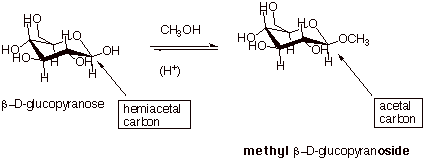
Disaccharides and Polysaccharides
q
We note that sugars have
alcohol functional groups of their own. There is therefore no need to supply an
alcohol function from a solvent such as methanol.
q
The –OH group of
one sugar molecule can serve as the alcohol function to replace the C1
–OH group of another molecule.
q
The most common case is
that the alcohol function is supplied by C4.
q
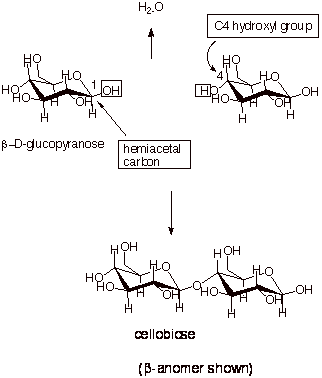
In the illustration
below, we see, first of all the formation of an acetal (or glycoside) linkage
by bonding the C4 –OH group of one b-D-glucopyranose
molecule to the C1 (hemiacetal) carbon of a second molecule of b-D-glucopyranose.
q
The resulting molecule
is called cellobiose. It is designated as a disaccharide, because it can be
hydrolyzed to two molecules of simpler sugars.
q
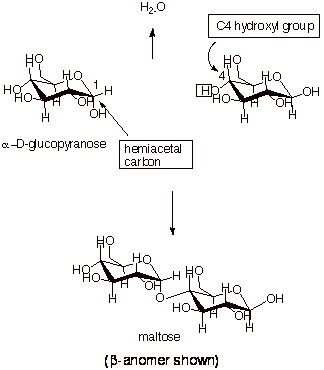
In a similar way, the
alpha anomer of glucopyranose can bond to another molecule of glucopyranose at
its C4 hydroxy group. The resulting disaccharide is called maltose.
q
Note that, since both
cellobiose and maltose have an acetal linkage, they can be hydrolyzed in
aqueous acidic solution to their component simple sugars (monosaccharides).
Both give two molecules of D-Glucopyranose (mixture of alpha and beta anomers:
remember, they equilibrate in aqueous solution.
q
Since maltose has one axial oxygen function, it is less thermodynamically stable
than cellobiose, which has all substituents equatorial.
q
The special importance
of the cellobiose/maltose examples is that they provide a pattern,
respectively, for the polysaccharides
cellulose and starch.
q
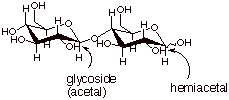
Finally, note that both
cellobiose and maltose have both a hemiacetal and an acetal function. You
should be able to find both.This is illustrated below for cellobiose.
Reducing Sugars. Aldehydes, via their specific aldehydic hydrogen (the
one directly attached to the carbonyl carbon) are uniquely easily oxidizable
amount carbon compounds. Even mild oxidants such as silver oxide and copper
oxide will oxidize an aldehyde to a carboxyl group (remember: +2 to +3
oxidation state). The metal oxide is then reduced to the metal, which is
revealed by its plating out on the reaction vessel.
q
Sugars which behave in
this way are called “reducing sugars”.
q
Reducing sugars are
those which are able to equilibrate with their free aldehyde form, because it
is only the aldehyde which reactions with the oxidant.
q
In alkaline solution,
recall that only hemiacetals, not acetals (or, the same thing, glycosides), are
able to equilibrate with the free aldehyde.
q
Consequently, only
sugars which have a hemiacetal carbon are “reducing sugars”. This
includes all of the monosaccharides and disaccharides mentioned so far.
q
By the way, since
mutarotation also requires the free aldehyde form, those same sugars which are
reducing sugars also exhibit mutarotation.
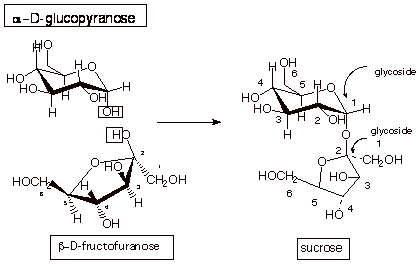
Sucrose.
The
structure of sucrose, a common disaccharide (table sugar) is shown below.
q
Sucrose corresponds to
linking a D-glucopyranose molecule as the alpha anomer and from its C1
hemiacetal hydroxyl group, with a
molecule of D-fructofuranose at its C2 (carbonyl) carbon. Note that the alcohol
function in this case comes from the hemiacetal OH of D-glucopyranose, not the
C4 normal alcohol function.
q
Note that this
disaccharide has no hemiacetal functions because C1 (the hemiacetal carbon)of
the D-glucopyranose molecule has been converted to a glycoside linkage. This
alcohol function has reacted with the acetal function of fructose.
q
Since this sugar has no
hemiacetal linkages, it cannot equilibrate with the free aldehyde form in
neutral or alkaline solution, so it is not a reducing sugar.
q
Similarly, it does not
display mutarotation in alkaline
or neutral solution.
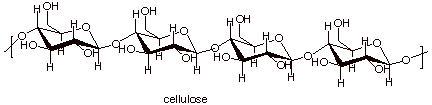
Polysaccharides.
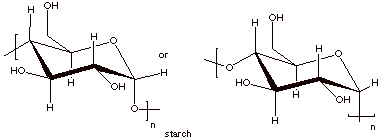
Cellulose, the important structural material of
plants, and starch, an important energy source for plants and animals, are both
examples of polysaccharides. They are high molecular weight molecules which
consist exlusively of D-glucose molecules bound together via their C1 and C4
carbon atoms.
q
Cellulose has a
structure which is essentially that of cellobiose, which was discussed before,
but with tens of thousands of glucose molecules instead of just two.
q
This means that they are
C1-C4 linked and at the C1 carbon, all of the linkages are of the beta type.
q
Repeat structures of
both starch and cellulose polymers are shown below.