INNER VS OUTER SPHERE ELECTRON TRANSFER MECHANISMS
The Outer Sphere
Mechanism. The term “outer sphere”
electron transfer refers to the transfer of an electron between two species or
(if intramolecular) between two functionalities without the incursion of
significant covalent bond formation between the two species or the two
functional groups. If the electron transfer is from a neutral species to a
cation radical, resulting in the formation of a new, neutral species and a new
cation radical, the specific type of electron transfer can be designated as
“hole transfer”. This terminology is based upon the view that the
cation radical moiety represents a vacancy or hole in the electron density of
the parent neutral molecule. In this case, substituent effects upon the
electron or hole transfer simply represent the effect of the substituent upon
the charge/spin density of the cation radical, assuming that the neutral
molecule is relatively nonpolar. The most direct way of investigating
substituent effects upon the stabilization of a cation radical relative to its
parent neutral is by measuring the oxidation potentials of a series of neutral
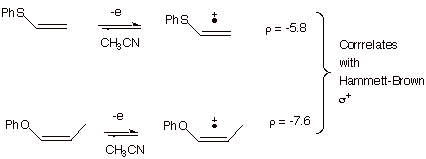
molecules having different substituents. In the case of the oxidation of a
series of aryl vinyl sulfides having different substituents on the aryl ring at
the para and/or meta positions, it is found that the oxidation potentials
directly correlate with the Hammett-Brown sigma values, indicating that there
is much positive charge generated directly upon the ring, i.e., that the entire
aryl vinyl sulfide pi electron system is an efficiently conjugated one
involving the pi electrons of the aryl ring, the sulfur 3p electrons, and the
vinyl pi bond. The rho value (-5.8) is quite large and negative, as would be
expected for the generation of a large amount of positive charge upon the ring.
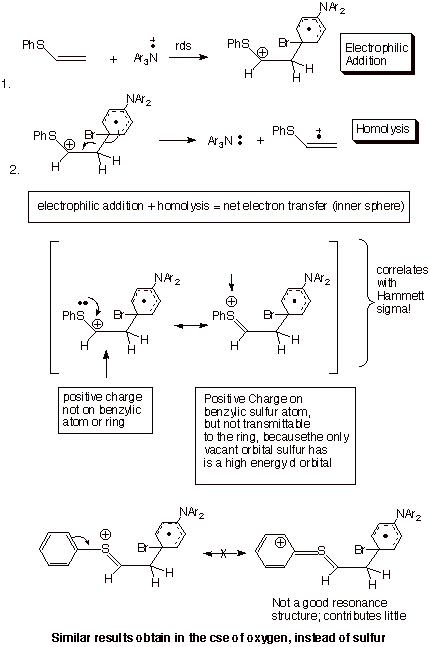
The Inner Sphere Mechanism
for the Ionization of Aryl vinyl sulfides
by Tris(4-bromophenyl)aminium hexachloroantimonate . An extreme form of the inner sphere
mechanism would be a two-step process in which a covalent bond between the two
interacting species is fully established, to yield a distonic cation radical.
If this electrophilic addition process is then followed by hemolytic
dissociation of the newly formed covalent bond, the net result is electron
transfer. This has in fact been established in the ionization of aryl vinyl
sulfides and cis-aryl propenyl ethers by tris(4-bromophenyl)aminium
hexachloroantimonate.
Incidentally, the reactions were studied observing the rate of formation of cation radical Diels-Alder and cyclobutanation reactions with appropriate electron rich molecules, in which the rds is the ionization of the sulfide or ether substrate (See Bauld,N.L.; Aplin,J.T.; Yueh,W.; Loinaz,A. J.Am.Chem.Soc. 1997, 119, 11381-11389.)
It
has been found also that the rates of protonation of these same ethers and
sulfides (both experimentally and calculationally) correlate with the Hammet
sigma values, as opposed to the Hammett-Brown sigma plus values (See
Bauld,N.L.; Aplin,J.T.; Yueh,W.; Endo,S.; Loving,A. J.Phys.Org.Chem. 1998, 11, 15-24.)