CH 610A/618A
Second Exam
Fall, 2002
Prof.N.L.Bauld
__________________________________________ Name (Last,
First)
__________________________________________ Social Security Number
Question |
Possible Points |
Page/Pts/Points Earned |
Score |
|
I. |
21 |
2/7/ 3/9/ 4/5/ |
|
|
II. |
22 |
5/16/ 6/6/ |
|
|
III. |
15 |
7/12/ 8/3/ |
|
|
IV. |
19 |
9/11/ 10/8/ |
|
|
V. |
23 |
11/14/ 12/9/ |
|
|
Totals |
100 |
|
|
I. Acids and Bases in Organic Chemistry
A. General Effects on Bronsted Acidity.
1. [3 pts] Consider the acid dissociation of a general acid
H-A in aqueous solution as written below. Cite the two general effects which are considered to influence the
relative acidities of acids where only the nature of A is varied. Indicate in
what sense (increase or decrease) they affect acidity and briefly explain why.
![]()
(1)
The bond strength
or bond dissociation energy (D) of the H-A bond. The stronger the H-A bond, the
less acidic is HA.
(2) The stability of the anion A-. The more
stable this anion is, the stronger the acid HA.
2. [2 pts each = 4 pts] For
each of the series given below, indicate in which direction acidity increases, which one of the general effects
you have mentioned is dominant,
and and supply an explanation of why this particular effect is dominant.
![]()
q
The acidity increases
from left to right, i.e., from HF to HBr. The bond strength effect dominates.
This is because the bond strength decreases greatly in a series which proceeds
down a column of the periodic table, but the anion stability varies only
slightly.
![]()
q
Acidity increases
from right to left, i.e., from methane to water. Anion stability dominates.
This is because in a series which proceeds across arrow of the periodic table,
bond strength varies relatively little, but electronegativity increases
tremendously toward the right side, so that anion stability increases greatly
as we go from methide to amide to hydroxide anion.
3. [3 pts each = 6 pts] For each of the pairs of Bronsted acids listed below, indicate the order of relative acidities , name the specific effect which is considered to cause this order of acidities, and explain the detailed basis for the effect, including a depiction of the effect..
. ![]()
q
Acetic acid is more
acidic than ethanol, because the anion of acetic acid, i.e. the acetate anion,
is resonance stabilized , but the anion of ethanol is not. In both anions, the
negative charge is on oxygen, so the resonance effect is essentially the only differentiating effect.
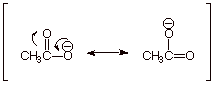
![]()
q
Acidity increases
from left to right, i.e., is greatest for ethyne and least for ethane.
q
This is because of a
hybridization effect. Thek
anion of ethyne has the unshared electron pair in an sp AO, which has 50%
s character, whereas the anion
from ethene has the unshared pair in an sp2 AO, which only has 33% s
character and that from ethene in an sp3 AO, which has 25% s
character.
q
Since a 2s AO is
substantially lower in energy than a 2p AO, the greater the percent s character
the lower in energy is the orbital.

![]()
q
Chloroacetic acid is more
acidic than acetic acid.
q
The general effect is
anion stability and the specific effect is termed an inductive effect. We
will also accept “field effect” or “dipolar effect”.
q
The C-Cl dipole of the
chloroacetate anion is oriented so that the positive end of the dipole (the
carbon end) is closer to the anionic oxygen centers than is the negative end of
the C-Cl dipole. Consequently, the attractive interaction is dominate and
results in stabilization of the anion by this inductive effect. .
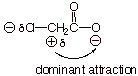
B. Acid/Base Equilibria
1. [3 pts] Consider the following acid/base reaction and
determine the position of the equilibrium using both a qualitative and a
quantitative criterion. State both criteria which you are using and show
precisely how they are applied.
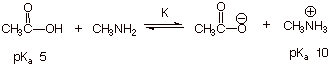
a. Qualitative Criterion: [1 pt]
q The
stronger acid is converted to the weaker acid.
q The
lower the pKa the stronger the acid, so acetic acid is stronger than
the methylammonium ion.
q The
equilibrium proceeds to the right.
b. Quantitative Criterion [2
pts]
q
![]()
q
The reaction proceeds toward the right.
q
C. Lewis Acids/Bases
1. [3 pt] For the reaction shown below, supply the
correct structure of the product and indicate which reactant species is the
Lewis acid and which the Lewis base. Then explain what specific aspect of each
reactant molecule enables it to function as the Lewis acid or base.
![]()
q
BF3
has a vacant 2p AO, which enables it to function as the Lewis acid or
electrophile.
q
NH3 has an
unshared electron pair, which enables it to function as a Lewis base or nucleop
2.
[2 pts] Consider the structure of the acid/base reaction product of the
reaction above. Could this molecule serve as a Lewis base? Why or why not?
Could it plausibly serve as a Bronsted acid? Why or why not?
q
The product could
not be an effective Lewis
base, because it lacks an
unshared electron pair or any other readily shared electron pair. Although the
boron is negatively charged, it has a filled second main shell and no other
electrons.
q
It could plausibly serve as a Bronsted acid, because there are acidic protons attached to the
positively charged nitrogen.
II. Stereochemistry
A. Definitions
1. [2 pt each = 6 pts] Provide
the appropriate stereochemical term corresponding to each of the following
definitions:
a. Stereoisomers which are not mirror images-- diastereoisomers
b. A molecule which has chiral centers but is achiral—a meso compound
c. The process of separating two mirror image isomers--resolution
B. Chiral vs. Achiral Molecules
1.
[2 pt each = 4 pts] Indicate
whether each of the following molecules is chiral or achiral and specify what
criterion you are applying in order to arrive at this conclusion.
![]()
q
The structure on the
left is achiral, since it
has no stereocenters. (The central carbon atom, e.g., has two ethyl groups
attached).
q
The structure on the
right is chiral because it
has a stereocenter, viz., the carbon which has OH, H, methyl, and ethyl groups
attached. Also, it has no other stereocenters, so it could not be meso.
C. Molecules Having Two Stereocenters
1. [4 pts] Draw the Newman projection structure of the
mirror image of the molecule shown below and designate its configuration using
the R,S nomenclature (you should specify the position locant of the R or S
configuration as is done in the name shown below (e.g. 3S,2R). Now draw the
Newman projection structures of the other two stereoisomers of
3-chloro-2-butanol and designate their configurations in the analogous way.
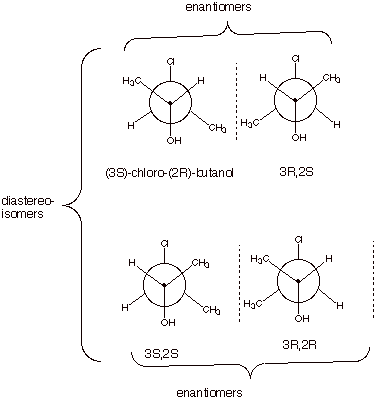
2.
[2 pts] Indicate the relationships between the various isomers, i.e., what type
of isomers they are with respect to each other. Are all of these molecules
chiral? Explain why or why not.
q
These are shown above.
q
Yes. Molecules with
stereocenters are only achiral if the stereocenters are equivalent. These
stereocenters are non-equivalent.
E. Separation of pure enantiomers from each other
1. [3 pts] Consider a 50:50 mixture of R and S-lactic acid (shown below as Fischer structures). Indicate the Fischer structures of the products (salts) which are formed when this mixture reacts (a simple acid/base reaction) with a single enantiomer (the R enantiomer) of 1-phenylethanamine (also shown below. Also, provide the stereochemical designations of each of the products (e.g., R,R or S,S etc.).
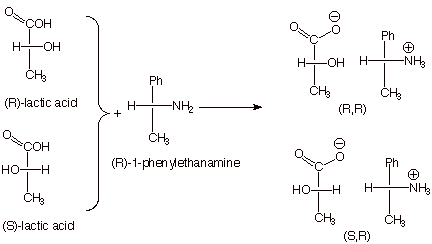
2.
[2 pts] What is the stereochemical relationship between these two products, and
how can they be separated? Explain why this separation method works.
q
They are
diastereoisomers.
q
They can be separated
by re-crystallization.
q
Since they are not
mirror images, they have different physical properties, including solubility.
3.
[1 pt] Draw the Fischer structure of the mirror image of one of these products.
Is it identical to either of the products? What is its stereochemical
designation?
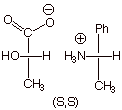
q
This is the mirror
image of the (R,R) product.
q
It is different from
either of the products of the original reaction.
III. Alkenes: Nomenclature and Stability
A. IUPAC Nomenclature
1. [3 pts] Provide the correct and complete IUPAC name for the following alkene, including any appropriate cis- or trans-designation:
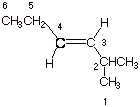
q
Trans-2-methyl-3-hexene. Incidentally, numbering
from the left side would give also give a 3-hexene, but the substituent methyl
group would then be at the 5-position (not acceptable).
2. [3 pts] Provide the correct and complete IUPAC name for the following alkene, including the designation as E or Z. In the latter connection, explain and show your priority rankings.
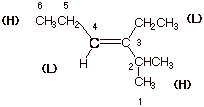
q
E-3-ethyl-2-methyl-3-hexene.
q
Note that there are
three different numbering systems that give a hexene and all three would be
3-hexenes, but only this one gives the first substituent at the 2-
position.
q
The ethyl group is
listed before the methyl because of alphabetics.
q
The high priority
groups are trans, so this is the E isomer.
q
Ethyl has a higher
priority than H, because C has a higher priority (atomic number) than H.
Isopropyl has a higher priority than ethyl, because although the alpha atoms
are carbon in both cases, the isopropyl group has 2 beta carbons, while the
ethyl has just one.
B. {3 pts] Provide the correct structure of the alkene which has
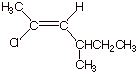
the IUPAC name Z-2-chloro-4-methyl-2-hexene.
C. Alkene Stability
1. [3 pts] Classify the following alkenes with respect
to their degree of substitution (mono-, di-, etc), and indicate their relative
order of thermodynamic stabilities (1 is the most thermodynamically stable).
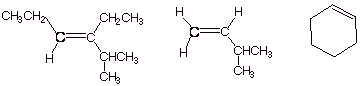
q
The first structure
on the left is trisubstituted.
It is the most stable.
q
The second structure
is monosubstituted. It is
the least stable.
q
The third structure
(cyclohexene) is disubstituted.
It is of intermediate stability.
D. Pi Bonding In Alkenes.
1. [3 pts] Illustrate by means of Newman projection
structures, and also showing the relevant overlap of 2p orbitals in pi bonding,
what happens when rotation around the C-C bond of cis-2-butene occurs to
ultimately give trans-2-butene. Is
this easy or difficult? How much of the pi bond remains at the 90 degree angle
of rotation? Explain.
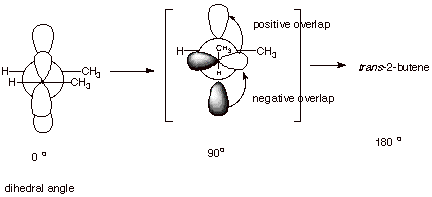
q
The overlap is weakened as rotation occurs around
the bond and vanishes completely at the 90 degree angle of rotation. Therefore,
this rotation is very hard to
do, since it completely breaks the pi bond.
q At the 90 degree angle of rotation, there is still some spatial overlap, but the amount of positive, bonding overlap just equals the amount of negative, antibonding overlap, and the net result is no bonding at all.
IV. Reaction Mechanisms:
General; Carbocation Intermediates
A. Definitions:
1. [2 pt each = 4 pts] Provide appropriate definitions for the following terms:
a. transition state – the highest energy structure on the lowest energy pathway of a reaction.
b. rate-determining step – a step of a multi-step reaction the rate of which is equal to the rate of the overall reaction.
B. Reaction Path Diagrams
1. Consider the following two step reaction:

a. [3 pts] Sketch a reaction path diagram which realistically
could represent such a reaction, indicating the position on the reaction
coordinate of R, I, and P and any transition states (TS).
q I can’t draw smooths path with my software,
but the identical reaction scheme and the corresponding path can be found in
your notes. It should be similar to the following scheme.
q The scheme also contains the answer to the b. part
of this question.
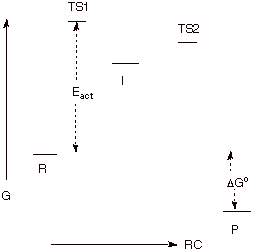
b. [2 pts] Indicate on the same diagram the energy
quantity which controls the rate of the reaction and also the energy quantity
which controls the position of an equilibrium between R and P, in the event
that it is established.
q The quantity Eact or activation energy
controls the rate of the reaction (the energy difference between R and TS1).
q The energy difference between P and R determines
the position of the equilibrium.
c. [2 pts] Cite, and illustrate on the diagram, two
specific conditions which must be satisfied in order for the first step to be
rigorously rate-determining.
q The intermediate must essentially always go on to
product, never back to reactants. This means that the barrier between I and TS1
(the activation energy for the reverse of step 1) must be greater than the barrier between I and TS2 (the
activation energy for step 2).
q The concentration of the intermediate must not
build up to a significant level, i.e., it must go on rapidly to the products.
This requires that the barrier to the second step be very low.
C. Carbocation Intermediates
1. [2 pts] Which of the carbocations depicted below, the methyl
carbocation or the ethyl carbocation is more thermodynamically stable and by
how much (approximately, in solution)?
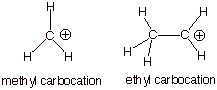
q The ethyl carbocation is more stable than the
methyl carbocation by about 15 kcal/mol in solution (much more in the gas
phase).
2. [3
pts] Provide a resonance theoretical description of the resonance stabilization
which exists in one of these carbocations. What specific sub-type of resonance
effect is this?
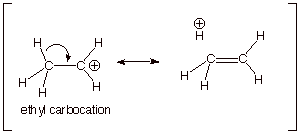
q This is called hyperconjugation.
3. [3
pts] Indicate the type of carbocation moiety (primary, secondary, or
tertiary) present in each of the
species shown below, and indicate
the order of stabilities, with 1 representing the most thermodynamically
stable.

q
The first, on the left, is a primary carbocation (it
has only one substituent, the cyclohexyl group). It is the least stable.
q
The middle structure is secondary, and is
intermediate in stability.
q
The last structure is tertiary, and is the most
stable.
V. Mechanism of H-Cl
Additions to Alkenes
A. The Reaction Mechanism.
1. [4 pts] Write the detailed reaction mechanism for the addition of H-Cl to ethene, employing all of the conventions of this course.
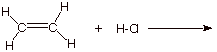
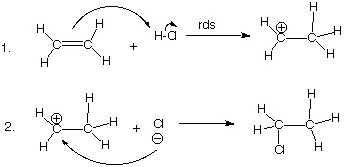
2. [2 pts] If one of the steps of this mechanism is rate-determining, explain in energetic terms why it is this step in particular which is rate-determining and not the other step.
q
The first step is rate determining. It involves breaking
two bonds and forming just one, so it is highly endothermic (endergonic) and slow. The second step involves breaking
no bonds and forming one, so it is highly exothermic and fast.
B. Transition State Models.
1. [5 pts] Provide a resonance theoretical treatment of the transition state for the rds of this reaction, summarize this as a DL/PC (dotted line/partial charge) structure, and characterize the TS as specifically as you can (without yet applying the Hammond Principle).
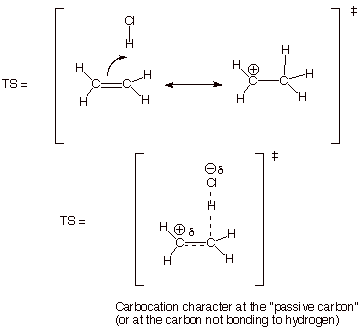
2. [3 pts] State the Hammond Principle, and use it to further refine your TS model, explaining the steps in your logic.
q The TS state of a reaction more strongly resembles the higher energy partner of the reactant or product.
q Since the product of step 1 is a very high energy carbocation intermediate, the TS of this step strongly resembles the carbocation intermediate.
q
Consequently we can now say that the TS has extensive
or highly developed carbocation character.
C. Selectivity.
1. [5 pts] Consider, now, the addition of H-Cl to propene. Using the TS model you have already generated, with appropriate slight modification for the case of propene, apply the Method of Competing Transition States to explain the preferred sense of addition of H-Cl to this unsymmetrical alkene (i.e., to which of the alkene carbons the proton adds).
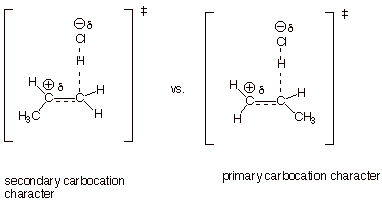
q
Since secondary carbocation character is much more
favorable than primary carbocation character, the first TS on the left is the
favored one.
2. [2 pts] Draw the structure of the predominant product of this reaction. What general term is used to describe this kind of selectivity or specificity (not looking for Markovnikov or anti-Markovnikov here)?
![]()
q
Regioselectivity or regiospecificity.
3. [2 pts] What is the symbolic mechanistic designation of this reaction?
q
AdE2