CH 610A/618A
Third Exam
Fall, 2003
Prof. N. L. Bauld
__________________________________________ Name (Last,
First)
__________________________________________ Social Security Number
Question |
Possible Points |
Page/Pts/Points Earned |
Score |
|
I. |
21 |
2/7/ 3/8/ 4/6/ |
|
|
II. |
17 |
5/9/ 6/8/ |
|
|
III. |
19 |
7/12/ 8/7/ |
|
|
IV. |
22 |
9/11/ 10/11/ |
|
|
V. |
11 |
11/11/ |
|
|
VI. |
10 |
12/10/ |
|
|
Totals |
100 |
|
|
I. Acid Catalyzed Additions to Alkenes
A. Acid Catalyzed Addition of Methanol to Isobutene.
1. [4 pts] Write the detailed mechanism for the reaction shown below. Be sure to
use the correct acid catalyst, which is the conjugate acid of methanol (the
methyloxonium ion). Assume that the first step is rigorously rate-determining.
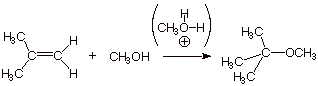
2.
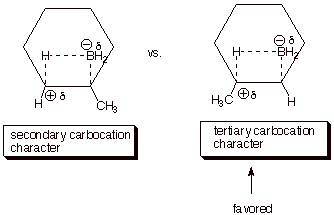
[3 pts] A DL/PC structure of the TS for the first (rate-determining) step is
provided below. Characterize this TS model as completely as you can without employing the Hammond Principle.
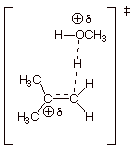
q
Carbocation Character
at the passive carbon
q
Tertiary carbocation
character.
3. [3 pts] State and apply
the Hammond Principle to this reaction step, explaining the energetic basis for your application.
q
The TS of a reaction
step more closely resembles the higher energy species of the reactant and
product.
q
The reaction involves
the breaking of two bonds (the pi bond and an O-H bond) and the formation of
just one bond (a C-H bond). It is therefore highly endothermic (or endergonic),
so the product of the step is the higher energy partner.
q
The TS therefore more
closely resembles the carbocation, which is the product of the step. We can say
that the TS has extensive (or highly developed) carbocation character.
B. Relative Rates of
Hydration of Alkenes.
1. [5 pts] Using the TS model previously provided for the
rds of the addition of methanol to isobutene, employ the Method of Competing
Transition States to discuss the
relative rates of addition of water to
ethene, propene, and isobutene (you should display all three TS models
and specify their respective characters). Which reaction is the fastest and
why?
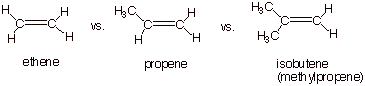
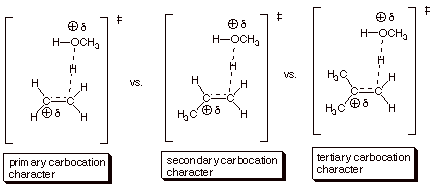
q
The reaction with
isobutene (the last one) is the fastest, because the TS has tertiary
carbocation character, which is much more favorable than secondary or primary
carbocation character.
C. Stereochemistry of
Additions to Alkene Double Bonds.
1. [3 pts] Name and illustrate the three possible stereochemical results in the addition of a general reagent (A-B) to an
alkene double bond.
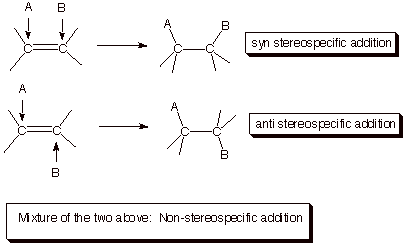
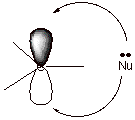
2. [3 pts] Which specific stereochemical result is typically observed in acid-catalyzed additions to
alkenes (e.g., acid-catalyzed hydration or addition of methanol)? Explain,
with the assistance of a mechanistic sketch, why this result is obtained. (You may use a general alkene and
hydronium ion as the catalyst).
![]()
q
Non-stereospecific
addition.
q
The intermediate
carbocation si sp2 hybridized (planar) and its 2p AO has two
equivalent lobes. The nucleophile can therefore react at either face of the
carbocation at equal rates.
II. Bromination
A. Bromination of Alkenes
1. [3 pts] Provide a full resonance theoretical treatment
of the epibromonium ion intermediate involved in the addition of Br2
to isobutene.
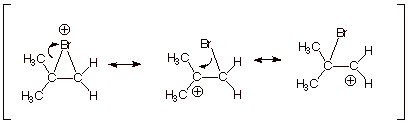
2. [3 pts] Provide the appropriate DL/PC model for this
intermediate and characterize it
fully in regard to the various locations of positive charge.
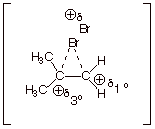
3. [3 pts] Upon which of the original alkene carbon atoms
is the positive charge greatest? Explain the basis for this.
q
On the tertiary
carbon.
q
The second resonance
structure (the one with positive charge on the tertiary carbon) is lower in
energy than the third resonance structure (which has positive charge on a
primary carbon). Consequently, the second resonance structure contributes more
than the third, and the real structure looks more like the second structure
than the third.
q
Therefore, there is
more positive charge on the tertiary carbon (because that is what the second
structure looks like).
4. [4 pts] Draw the structure of the predominant product
of the reaction of this epibromonium ion with water. Explain the basis for the
selectivity of the water nucleophile in reacting at one specific carbon in
preference to the other.
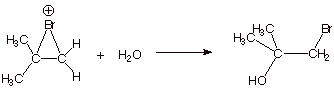
q
Carbocations tend to
react with nucleophiles at the position of highest carbocation character, which
is the tertiary position.
5. [4 pts] Draw the structure of the product of the reaction of bromine with
cyclohexene. Specify the reaction stereochemistry, and explain the basis for this particular stereochemical result.
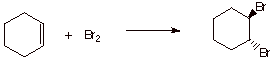
q
Anti stereospecific
addition.
q
The intermediate
epibromonium ion tends to react with the bromide ion nucleophile from the
opposite face from the bromine bridge (in effect, the bridging bromine blocks
the syn face and permits reaction only at the anti face).
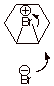
III. Hydroboration/Oxidation
A. Hydroboration
1.
[5 pts] Draw the structure of the
major product of the reaction of propene with borane, provide a resonance
theoretical treatment of the TS for
the formation of this product, summarize as a DL/PC model, and characterize the TS very carefully.
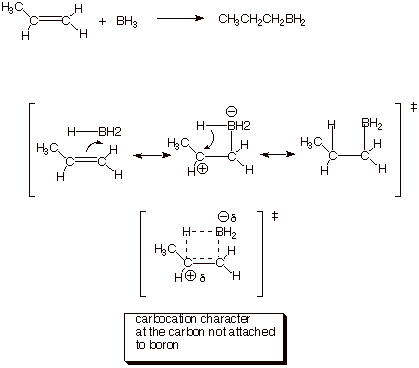
3. [2 pt] What character of this TS is not present in
either the reactant or the product? Explain why it is appropriate to include a
non-reactant-like and non-product-like structure in the resonance treatment.
q
The carbocation
character.
q
Resonance theory calls
for the inclusion of all energetically reasonable structures, not just reactant
and product.
4. [3 pts] Use the Method of competing TS’s to rationalize the orientation of the addition of borane to propene. Your discussion should include TS models and characters for both modes of addition to propene. Indicate which TS is preferred and explain why.
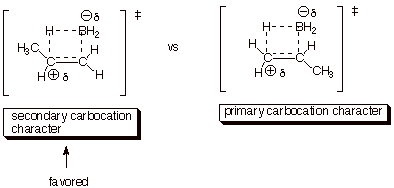
5. [2 pts] Provide the name of and briefly explain a
second factor which favors the addition of borane to propene in the preferred
manner.
q
Steric effect. In the
favored TS the boron approaches a less sterically hindered primary carbon,
whereas in the less favored TS the boron approaches a somewhat more hindered
secondary carbon atom.
6. [2 pt] What term is used to indicate the kind of
orientational selectivity observed in this addition to propene?
q
Regioselectivity (or
regiospecificity).
7. [2 pts] How many steps are involved in the
hydroboration of an alkene? What specific term is used to describe a reaction
having this number of steps?
q
One step.
q
Concerted.
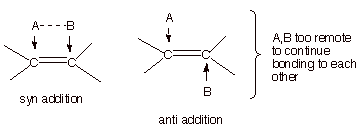
7. [3 pts] What stereochemical term is used to describe the stereochemistry of
hydroboration? Rationalize, using an appropriate illustration, why this particular stereochemical result prevails.
q
Syn stereospecific
(both terms required for full credit).
q
When two atoms which
are attached (bonded) to each other add to an alkene pi bond, they are
physically unable to maintain their bonding to each other while attaching to
opposite faces of the double bond, but they can access the same face.
IV. Radical Stability;
Radical Chain Reactions: Halogenation of Alkanes
A. Radical Stability
1. [3 pts] Provide a resonance theoretical treatment of
the ethyl radical (see below). Which canonical structure is of lower energy?
Why?

q
The first structure
is lower in energy. It has a C-H sigma bond, which is stronger than a C-C pi bond.
2. [2 pts] What does this treatment imply about the distribution of the odd electron
(radical character) in the ethyl radical (where is it and where is it the
greatest?)? Explain.
q
It is partially on
the right hand carbon atom (the methylene carbon) and partially on the beta
hydrogens (the methyl hydrogens).
q
It is greatest on the
carbon atom because the first structure, which has the odd electron on carbon,
is the lower energy structure. The real structure more closely resembles the
lower energy structure.
B. Radical Structure
1. [2 pts] Draw the structure of the methyl radical (see
below), indicating the geometry of the radical and the hybridization of the
carbon atom.
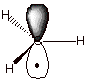
q
The hybridization of
carbon is sp2, so all four atoms are coplanar.
2. [1 pts] What type of AO does the odd electron occupy?
q
A 2p AO.
B. Halogenation of Methane
1.
[3 pts] Thermodynamic Considerations
a. Provide an equation for approximating the standard enthalpy change (DHo) of a reaction and apply it to the chlorination of methane, using the bond dissociation energies provided.
![]()
Bond Dissociation Energies: D(H-Cl) = 103 kcal/mol; D(CH3-Cl) = 86 ; D(CH3-H) = 105 ; D(Cl-Cl) = 58.
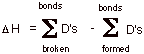
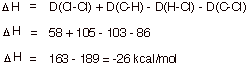
2.
The Reaction Mechanism
a.
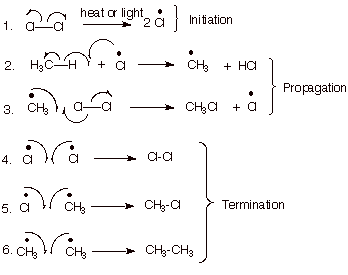
[5 pts] Write the complete, detailed mechanism for the chlorination of methane,
specifying the three stages of the reaction.
C. Transition State Models
1. [3 pts] Provide a resonance theoretical description of the transition state of the reaction in which chlorine atoms remove a hydrogen atom from methane, summarize this as a dotted line/partial radical character structure, and characterize the TS model.
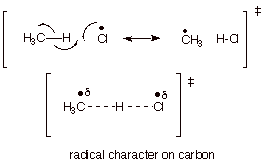
2. [3 pts] Extend the DL/PRC
structure from the previous question to the case of bromination (abstraction of
a hydrogen atom by bromine atoms), and apply the Hammond Principle to refine your TS characterization. Use this refined
characterization to explain why bromination is highly selective for tertiary C-H bonds.
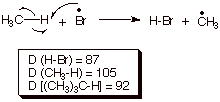
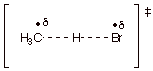
q
The abstraction step
is highly endothermic when bromine atoms are doing the abstracting. This
follows because the bond broken has a D = 105 kcal/mol, while D = 87 for the
HBr bond which is formed. From the formula previously given, the enthalpy
change for this step is +18 kcal/mol.
q
Applying the Hammond
Principle, the TS for this abstraction closely resembles the product of the
step, which is the radical (in this case the methyl radical). Thus, the extended TS character is highly developed
radical character. The abstraction rate depends sensitively upon the stability
of the radical (tertiary more stable than secondary than primary).
V.Radical Additions to
Alkenes; Grignard Formation
A. Radical Chain Additions to Alkenes
1. [5 pts] Write the detailed mechanism for the radical chain addition of HBr to propene, labeling the three distinct stages of the reaction, and specifying the radical conditions that are needed to bring about this reaction.
![]()
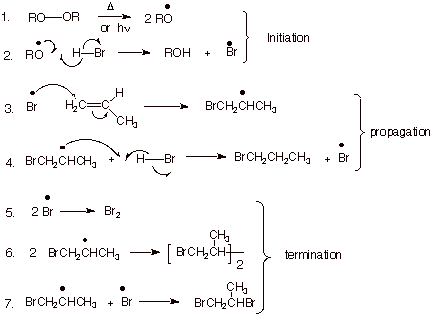
q
Radical conditions consist of using a peroxide
(ROOR) and either heating or using uv light.
B. Grignard Formation.
1. [3 pts] Write the detailed
mechanism for the formation of the Grignard reagent shown below.
![]()
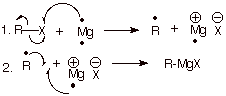
3. [3
pts] Describe the bonding in the Grignard
reagent, including both the C-Mg and Mg-X bonds. What kind of reactivity does
the carbon atom of a Grignard reagent possess?
q The
C-Mg bond is polar covalent and
the Mg-X bond is largely ionic.
q The
carbon of a Grignard reagent is nucleophilic.
V. Syntheses
A. Product Structures
1. [2 pts each = 8 pts] Draw the complete structure(s) of the product (or all of the main products if more than one product is formed in substantial amounts), including the stereochemistry of the products. No explanations are necessary in this part of the question.
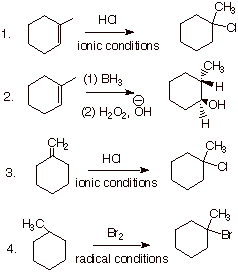
2. [2 pts] In example 2 of the above question (hydroboration of 1-methylcyclohexene), explain, using the method of competing TS’s, the theoretical basis for the preferred regiochemistry of the reaction.