CH 610A/618A
Third Exam
Fall, 2002
Prof. N. L. Bauld
Answer Key
__________________________________________ Name (Last,
First)
__________________________________________ Social Security Number
Question |
Possible Points |
Page/Pts/Points Earned |
Score |
|
I. |
21 |
2/13/ 3/8/ |
|
|
II. |
27 |
4/9// 5/18/ |
|
|
III. |
21 |
6/7/ 7/14/ |
|
|
IV. |
21 |
8/12/ 9/9/ |
|
|
V. |
10 |
10/10/ |
|
|
Totals |
100 |
|
|
I. Acid Catalyzed Hydration of Alkenes
A. Acid Catalyzed Hydration of Ethene (Addition of Water)
1. [4 pts] Write the detailed mechanism for the reaction shown below, i.e. the acid
catalyzed hydration of ethene.
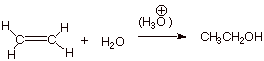
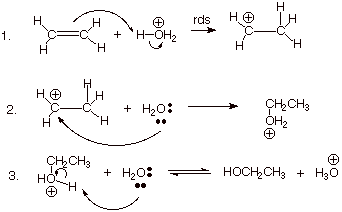
[Please note: Requires rds for first step and
equilibrium arrows for last step, along with all other conventions for writing
mechanisms.]
2.
[5 pts] Provide a resonance theoretical description of the transition state for
the rate determining step of this reaction, summarize this as a DL/PC structure
and characterize this TS model as completely as you can without employing the
Hammond Principle.
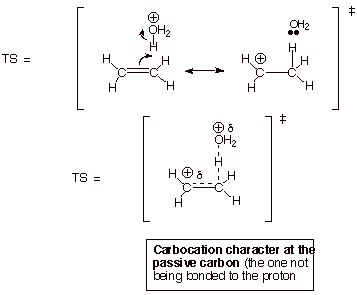
3. .
[2 pts] State and apply the Hammond Principle to this reaction step, explaining
your application.
q
The TS for a reaction
step more closely resembles the higher energy partner of the reactants and
products
q
The rds is highly
endothermic, since two bonds are broken (the C-C pi bond and an O-H bond) and
one (the C-H bond) is formed.
q
Therefore, the TS
more closely resembles the product of the reaction step, which is the
carbocation.
q
The TS thus has
extensive (or highly developed) carbocation character at the passive carbon.
4. [2 pts] Now, apply the Hammond
Principle to the step of the reaction which involves the reaction of the
intermediate carbocation with water. What predominant character does this TS
have?
q
The reaction of the
carbocation with water is highly exothermic, since one bond is formed (the C-O
bond) and none are broke.
q
The TS therefore more
closely resembles the reactant, which is the carbocation.
q
Therefore this TS
also has extensive carbocation character.
B. Relative Rates of
Hydration of Alkenes.
1. [4 pts] Using the TS model you have
previously derived for the rds of the hydration reaction, employ the Method
of Competing Transition States to
discuss the relative rates of addition of water to ethene, propene, and isobutene (you should display all three
TS models and specify their respective characters). Which reaction is the
fastest and why?
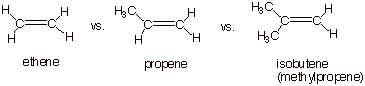
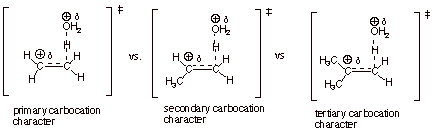
q
The reaction with
isobutene is fastest, because the TS has tertiary carbocation character, which
is more favorable than secondary or primary carbocation character.
C. Stereochemistry of Hydration.
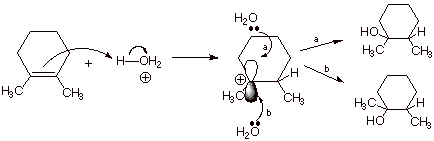
1. [4
pts] Sketch the mechanism of the
acid-catalyzed hydration of 1,2-dimethylcyclohexene in such a way as to
illustrate and explain the observed stereochemistry of the reaction. Write the
structure(s) of the product(s) observed and indicate what stereochemical term
is used to describe this kind of stereochemical result. (Note that the
“squiggly” line means that I have deliberately left the
stereochemistry unspecified).
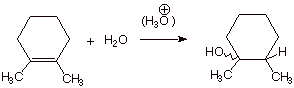
q
The reaction is non-stereospecific.
q
The 2p AO of the
intermediate carbocation has equivalent lobes above and below the carbocation
center and can react with water from either face, yielding both the cis and
trans products.
II. Bromination and Hydroboration/Oxidation
A. Bromination of Alkenes
1. [3
pts] Write the structure(s) of the product(s) obtained in the following
reaction. What terminology is used to describe this kind of stereochemical
result (two words)?
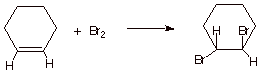
q
The reaction is is
termed anti stereospecific (only
the trans-product is obtained.)
2. [3 pts] Rationalize, with the
assistance of a mechanistic sketch, why this particular stereochemical result
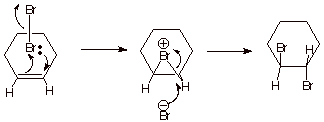
is observed. What is the generic name of the type of intermediate involved?
q
The reaction involves
an epibromonium ion
intermediate (alternatively, a bridged bromonium ion).
q
Since one face
(assume the top face) is blocked by the bridging bromine, the bromide ion
reacts from the opposite face, leaving one bromine or the top face and one on
the bottom face [an alternative, and better, explanation is that SN2
reactions, like this one, occur with inversion of configuration at carbon, so
that the entering nucleophile always bonds from the opposite face from which
the leaving group leaves]
3. [3 pts] Draw the Newman projection structure of the dibromide(s)
which result(s) when bromine is added to trans-2-butene. Provide the complete IUPAC name of any
stereoisomer formed, including the stereochemical designation.
q
The product is
meso-2,3-dibromobutane or (2R,3S)-dibromobutane or (2S,3R)-dibromobutane.
q
Note that other
conformations, including eclipsed ones are acceptable as long as they depict
the meso diasteroisomer.
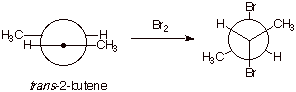
B. Hydroboration
1.
[5 pts] Provide a resonance theoretical treatment of the TS for the
hydroboration of ethene, summarize as a DL/PC model, and characterize the TS
very carefully.
![]()
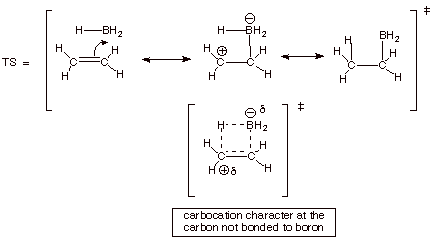
2. [1 pt] What character of this TS is not
present in either the reactant or the product? Carobocation character.
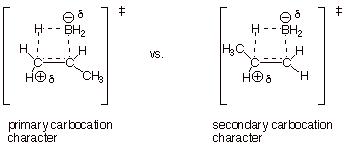
3.
[5 pts] Use the Method of competing TS’s to rationalize the orientation
of the addition of borane to propene, using and extending the TS model that you
developed for the hydroboration of ethene. Your discussion should include TS
models and characters for both modes of addition to propene. Indicate which TS
is preferred and explain why. Finally, draw the structure of the preferred
product.
q
The second TS is
favored, because secondary carbocation character is better than primary
carbocation character.
q
The preferred product
is propylborane, CH3CH2CH2BH2.
4. [2 pt] What term is used to indicate
this kind of selectivity?
q
Regioselectivity or
regiospecificity.
5. [2 pts] How many steps are involved in
the hydroboration of an alkene? What specific term is used to describe a
reaction having this number of steps?
q
It is a single step
reaction.
q
The appropriate term
is “concerted”.
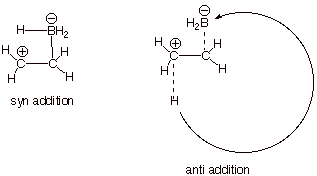
6. [3 pts] What stereochemical terminology
is used to describe the stereochemistry of hydroboration? Rationalize, using an
appropriate illustration, the reason that this stereochemical result prevails.
q
It is syn
stereospecific.
q
If the H attempts to
bond to the alkene from the opposite face, it cannot be simultaneously bonding
to boron, because the latter is too remote to bond to it. Only the syn face is accessible.
III. Radical Stability; Radical Chain Reactions: Halogenation of Alkanes
A. Radical Stability
1. [3 pts] The bond dissociation energies of the C-H bonds of methane
and ethane are 105 and 98 kcal/mol, respectively. Since both bonds are are of the
same type (C-H bonds in which the carbon is sp3 hybridized in both
cases), explain why one bond is more easily dissociated than the other. Your
answer should include a resonance theoretical treatment of one of the radicals
involved.
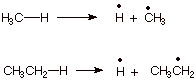
q The ethyl radical is resonance stabilized, as shown
below, but the methyl radical is not.

B. Halogenation of Methane
1.
[3 pts] Thermodynamic Considerations
a. Provide an equation for approximating the standard enthalpy change (DHo) of a reaction and apply it to the chlorination of methane, using the bond dissociation energies provided.
![]()
Bond Dissociation Energies: D(H-Cl) = 103 kcal/mol; D(CH3-Cl) = 86 ; D(CH3-H) = 105 ; D(Cl-Cl) = 58.

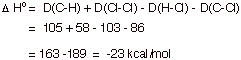
b. [1 pt] In an overall sense, what kind of reaction is this (the name of the reaction type)?
q
It is a homolytic substitution reaction or radical
substitution reaction.
2.
The Reaction Mechanism
a.
[5 pts] Write the complete, detailed mechanism for the chlorination of methane,
specifying the three stages of the reaction.
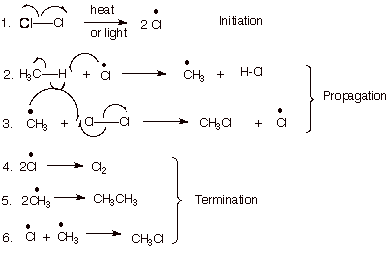
b.
[2 pts] What terminology is used to describe a reaction mechanism in which one
stage is repeated over and over again? Provide, in addition, the symbolic
mechanistic description of the reaction.
q
A chain (or radical
chain) reaction.
q
It is an SH
or SR reaction. (substitution, hemolytic or radical)
C. Transition State Models
1. [5 pts] Provide a resonance theoretical description of the
transition state of the reaction in which chlorine atoms remove a hydrogen atom
from methane, summarize this as a dotted line/partial radical character
structure, and characterize the TS model.
![]()

2. [2 pts] What is the term
used to describe a reaction which involves the removal of a H atom by a
chlorine atom to form an H-Cl bond (or any analogous radical process)?
q
It is an abstraction
reaction.
IV.Radical Additions to
Alkenes; Grignard Formation
A. Radical Chain Additions to Alkenes
1. [4 pts] Write the detailed mechanism for the radical chain addition of HBr to ethene, labeling the three distinct stages of the reaction, and specifying the radical conditions that are needed to bring about this reaction.
![]()
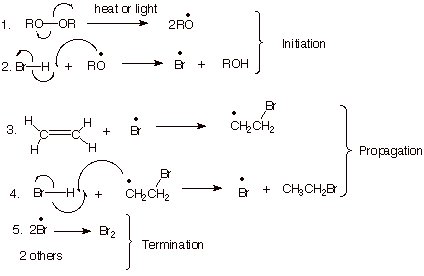
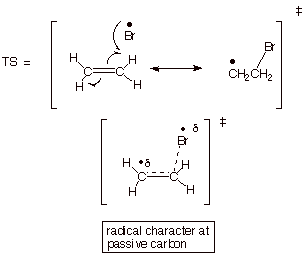
2. [4 pts] Provide a resonance theoretical treatment for the TS for the addition of bromine atoms to ethene and summarize as a dotted line/partial radical character structure.
![]()
3. [4 pts] Using this dotted line/ partial radical character TS model and extending it to the case of addition of HBr to propene, employ the Method of Competing TS’s to compare the TS characters of the two possible modes of addition of bromine atoms to propene. Indicate which mode is preferred and why.
![]()
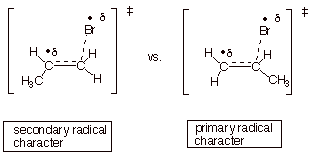
q
Secondary radical character is better than primary
radical character.
q
The first path is preferred, and this gives
1-bromopropane.
B. Grignard Formation.
1. [3 pts] Write the detailed
mechanism for the formation of the Grignard reagent shown below.
![]()

2. [1 pt] The mechanism is, of course, a radical mechanism, but
it differers in a basic respect from the other radical reactions we have
studied. In what general mechanistic respect does it differ from these others?
q This is not a chain reaction, it is a
stoichiometric or non-chain
radical reaction.
3. [3 pts] Describe the bonding in the Grignard reagent,
including both the C-Mg and Mg-X bonds. What kind of reactivity does the carbon
atom of a Grignard reagent possess?
q The C-Mg bond is polar covalent , with carbon being the negative end of the dipole.
q Carbon displays nucleophilic reactivity.
4. [2 pts] Mention and briefly explain two respects in which
diethyl ether is an especially good solvent for the preparation of Grignard
reagents.
q Ethers are non-acidic, so they do not protonate the
quite basic Grignard reagents, i.e., Grignard reagents are stable in ethers
because of this lack of basicity.
q The C-O dipole of ethers, in which oxygen is the
negative end, allows ethers to strongly stabilize the Mg positive ion, and thus
to dissolve Grignard reagents. (Also equally acceptable, the unshared electron
pairs in ethers makes them fairly good Lewis bases, which enables them to
stabilize Mg positive ions, which are Lewis acids.
V. Syntheses
A. Product Structures
1. [2 pts each = 10 pts] Draw the complete structure(s) of the product (or all of the main products if more than one product is formed in substantial amounts), including the stereochemistry of the products. No explanations are necessary.
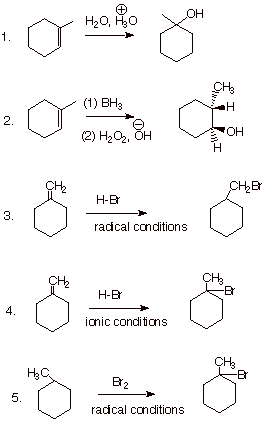
q Note that the only stereochemical issue is in part 2, where the OH and the H are cis, because we have syn stereospecific addition of borane, etc.