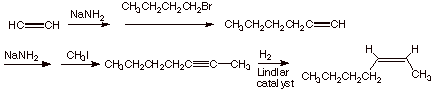CH 610A/618A
Final Exam
Fall, 2003
Prof. N. L. Bauld
__________________________________________ Name (Last,
First)
__________________________________________ Social Security Number
Question |
Possible Points |
Page/Pts/Points Earned |
Score |
|
I. |
25 |
2/9/ 3/9/ 4/7/ |
|
|
II. |
26 |
5/11/ 6/7/ 7/8/ |
|
|
III. |
29 |
8/11/ 9/10/ 10/8/ |
|
|
IV. |
15 |
11/9/ 12/03/ 13/03/ |
|
|
V. |
22 |
14/13/ 15/9/ |
|
|
VI. |
15 |
16/7 17/8/ |
|
|
VII. |
18 |
18/11 19/7/ |
|
|
Totals |
150 |
|
|
I.Mechanisms of Nucleophilic Substution Reactions
A. Nucleophilic Substitution Mechanisms
1. [4 pts] Write the detailed mechanism for the reaction shown below and give the
symbolic classification of the reaction. What does the numeral signify?
![]()
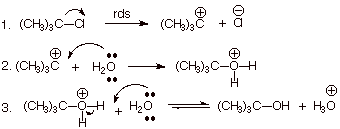
q
Symbolic Classification
of the Mechanism: SN1
q
Significance of the
numeral: The molecularity of (no of molecules in) the rds.
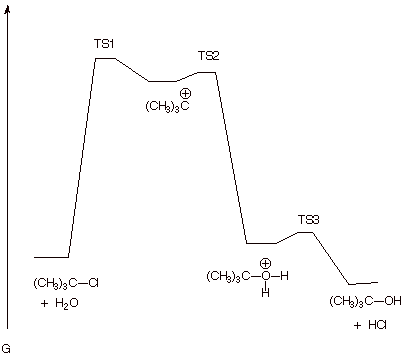
2. . [5 pts] Draw a reaction
path diagram for the reaction above
that is consistent with the mechanism you wrote. On your diagram, draw the
structures of any intermediates involved on this reaction path.
3. [4 pts] Provide a resonance theoretical treatment of the transition state for the rate determining step of the reaction, summarize as a dotted line/partial charge structure and characterize the TS model.
q
Resonance Treatment:
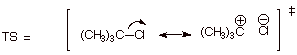
q
DL/PC:
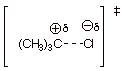
q
Characterization of TS
Model:
o
Carbocation character
4. [3
pts] State
the Hammond Principle, explain the application to this rds, and further refine
the TS characterization based upon this reasoning.
o
The transition state
of a reaction step more closely resembles the higher energy partner of the
reactant and product.
o
The rds breaks one
bond and forms none, so it is highly endergonic (or endothermic). Thus the
TS closely resembles the product
of the step, which is the tert-butyl carbocation.
o
Therefore the TS has
extensive carbocation
character.
5. [2 pts]
Consider the analogous reaction of 2-chloropropane with water
to give 2-propanol. What mechanism is operative in this reaction? Explain, in
detail, what considerations allow us to confidently make this prediction.
![]()
q
This is a secondary
halide, so the SN reaction could proceed via either of the two
available mechanisms. Which one is followed depends upon the strength of the
nucleophile. Stronger nucleophiles favor the SN2 mechanism, because
the nucleophilie participates in the rds in this mechanism, whereas it
doesn’t in the SN1 mechanism.
q
Water is a very weak
nucleophile, so this reaction prefers the SN1 route.
6. [3 pts]
Use the Method of Competing Transition States
(provide illustrations of the rds’s of both TS’s) to derive a
prediction about which of these two reactions (that of tert-butyl chloride or isopropyl chloride) is the fastest.
Explain your reasoning.
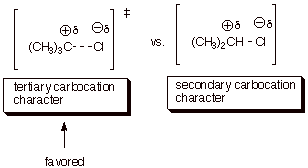
7. [2 pts] Is the difference in rates likely to be large or small? Explain in detail the basis for your prediction.
q
Large,
because the carbocation character is extensive. Therefore the difference between the
stabilization of a tertiary carbocation vs. a secondary carbocation, which is
large, will be largely observed in the TS.
8. [2 pts] Since hydroxide anion is a much stronger nucleophile than water, why was the former not used instead of water? Give two distinct reasons.[ Hint for one of the reasons: What is the major product in the reaction of tert-butyl chloride with hydroxide ion?]
![]()
q
Hydroxide ion is also
a strong base, and can engender elimination reactions.
q
Since the tertiary
system will still only undergo an SN1 reaction, the stronger
nucleophilicity of hydroxide ion will not enhance the rate at all.
q
The product is
isobutene, from E2 elimination.
II. More On Nucleophilic Substitutions
A. Consider the nucleophilic substitution reaction given in the equation below:
![]()
1. [2
pts] Based upon the structure of the alkyl halide involved in this reaction,
you should be able to predict the kind of mechanism involved. What is the
mechanistic designation for this reaction?
q
An SN2
mechanism.
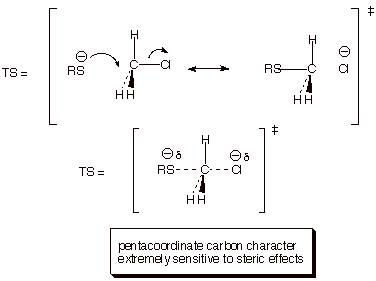
2. [5 pts] Provide a resonance theoretical
treatment for the rds of this reaction, summarize as a DL/PC structure,
characterize the TS, and indicate what kind of structural effect this character
is especially sensitive to.
3. [2 pts] Write down the form of the kinetic rate law for
this reaction, and define the term “rate constant”.
![]()
q
The rate constant is
equal to the rate of the reaction at unit concentrations of all reactants.
4. Now
consider the following analogous reaction:
![]()
q
[2 pts] What mechanism of substitution is expected to operate
here? Explain in detail.
q
The SN2
mechanism.
q
Again, secondary
systems are “swing systems”, and can proceed via either mechanism.
The preferred mechanism depends largely upon the strength of the nuclephile.
q
The anion in this
reaction is an extremely strong nucleophile, so the reaction prefers the SN2
mechanism.
q
[2 pts] Is elimination expected to be a major competing
problem in this case? Explain why or why not.
q
No. Because the
nucleophile is very strong, but is not a very strong base.
1. Solvent Effects
1.
[3 pts] Using the DL/PC model of the TS for the rds of the reaction of
chloromethane with the alkylthiyl anion (II.A.2, above), derive a prediction of
the effect upon the reaction rate of this reaction when the solvent polarity is
increased. Be specific, and explain your reasoning.
q
The TS has the
negative charge delocalized over the whole TS, including the S atom and the
chlorine atom. This anion has a much larger volume than the starting sulfide
anion.
q
Larger anions are
less efficiently solvated, so the transition state benefits less from the
solvation of the more polar solvent than does the reactant anion. [Put another
way, the reactant anion is more stabilized by the solvent than is the TS]
q
So the reaction is
slowed down by a modest amount (modest because charge is not being created or
removed).
2.
[2 pts] Finally, suppose that the solvent in that same reaction is changed from
a
hydroxylic
solvent (like methanol) to dimethyl formamide or dimethylsulfoxide. What kind
of effect does this have upon the rate? Explain, and name the type of
solvent represented by these two examples.
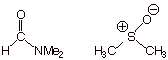
q
The reaction will
will be greatly speeded up
by using a dipolar aprotic solvent, such as DMF or DMSO.
q
This is because the
sulfide anion reactant is powerfully stabilized by hydrogen bonding in a
hydroxylic solvent such as
methanol. Therefore it is less reactive as a nucleophile. However, in a dipolar
aprotic solvent, the anion is relatively weakly solvated (“naked”)
and is therefore very reactive.
C. Stereochemistry.
1.
[ 3 pts] Sketch the mechanism of the reaction shown below so as to illustrate the
stereochemical outcome of the reaction. Draw the structure(s) of the product(s)
and provide the term which
describes this stereochemical outcome. [Again, the “squiggly line
indicates an unspecified stereochemistry, which is for you to specify.]
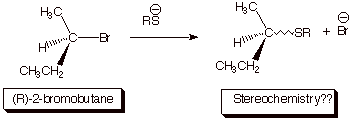

D. Leaving Group Ability
1.
[ 2 pts] Hydrogen bromide (HBr) is a significantly stronger acid than hydrogen
chloride (HCl). Using this observation, predict whether bromide anion or
chloride anion is a better leaving group in nucleophilic substitution
reactions. Explain the basis for your conclusions.
q
Bromide ion is a
better leaving group.
q
Leaving group ability
correlates with weak basicity, and bromide ion is a weaker base than chloride
ion.
E. Nucleophilicity.
1. [ 3 pts] Predict which of the following reactions is
fastest and explain your reasoning in detail. Provide the name of a specific
effect which is most relevant to this explanation.

q
The reaction with the
sulfur anion is much faster, because sulfide anions are much better
nucleophiles than alkoxide anions.
q
The basis for this is
usually considered to be polarizability. Sulfur, being a larger atom, has valence shell
electrons which are much more loosely held than oxygen. Consequently, they
distort and adapt in the crowded SN2 TS to minimize repulsions.
III.Elimination Reactions;
Competition with SN Reactions
A. Mechanism of Elimination
Reactions
1. [4 pts] Use resonance theory to derive a TS model
for the elimination reaction shown below, summarize as a DL/PC structure, and
characterize the resulting TS model.

q The TS has “alkene character”.
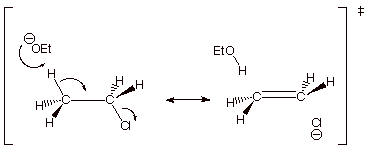
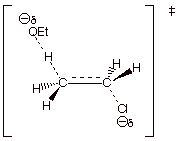
2.
[1 pt] Assuming that this is a
concerted reaction, what is the symbolic mechanistic designation for the
reaction mechanism?
q E2.
3.
[2 pts] Is this reaction base catalyzed? Why or why not? What is the
appropriate term to describe the role of the base in this reaction?
q Not base-catalyzed.
q The base is consumed stoichiometrically in the
reaction.
q Base-Promoted.
4.
[1 pts] What term is used to describe the role of chloride ion in this
reaction?
q Leaving Group.
B. Selectivity in Systems in
Which There are Two Non-Equivalent Beta Hydrogens
1. [3 pts] Provide the structures and names of the products of the following reaction. Designate the alpha and the two distinct beta positions of the reactant and indicate which product arises from the removal of which beta hydrogen, and which product is the major product.
![]()
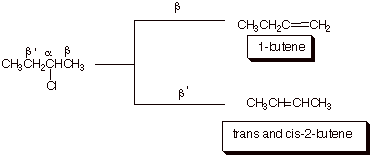
2.
[4 pts] Use
the Method of Competing Transition States to compare the TS’s for the
reactions leading to these products. Apply the respective characters of these transition
states to explain the predominance of the product which is observed to be major.
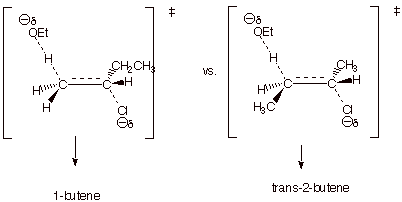
q
The first TS has
monosubstituted alkene character; the second has disubstituted alkene
character.
q
The latter is more
energetically favorable. Therefore 2-butenes are the major products.
3.
[2 pts] The selectivity in these eliminations is seen to be relatively low,
i.e., both products are formed in very substantial amounts. Provide two
distinct reasons for this lack of selectivity. One of these reasons should involve
an application of the Hammond Principle.
o
The reaction involves
the breaking of two bonds and the formation of two bonds. It is therefore not
highly exergonic nor highly endergonic. So the TS has only a moderate amount of
alkene character (not extensive).
o
The difference in
stability between a monosubstituted and a disubstituted alkene is not very
large, anyway (ca. 2.7 kcal/mol). So there is no possibility that energy
differences are going to give a very pronounced selectivity.
C. Stereochemistry of Eliminations
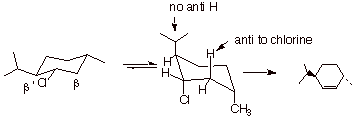
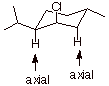
1. [4 pts] Draw the structures of the products of the following
eliminations and explain, with the assistance of conformational depictions, any
selectivities which may be involved. What is the preferred stereochemistry in
these reactions ( the relationship of the beta hydrogen and the leaving group)?
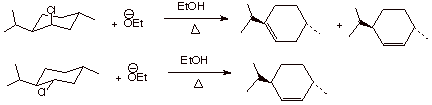
q
The first reaction
gives a mixture of alkenes, with the trisubstituted (more stable) alkene in the
majority. The second gives the less thermodynamically stable disubstituted
alkene with high selectivity.
q
The preferred
stereochemistry is anti
(180o dihedral angle). This is possible only when the leaving
group chlorine is axial and the beta hydrogen is also axial. In the second chloride, this is only achieved when
a ring flip occurs to give
an axial chlorine. The disubstituted alkene is formed because only at the beta
carbon is there an axial hydrogen.
q
In the case of the first chloride,no
ring flip is required because the chlorine is already axial and both thebeta
and beta prime carbons have axial hydrogens, so both alkenes can be formed. The
TS with the more stable alkene character is somewhat favored.
D. [6 pts] Predict whether each of the elimination reactions shown below is able to proceed.[Do not
consider any reaction other than elimination] If the reactions do not proceed,
explain why not. If they do proceed, provide the structure of the major
product, and explain why it is the major product.
1. 
q Does not proceed! The hydroxide ion is a poor leaving group.
2.
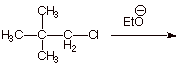
q Does not proceed! There is no beta hydrogen for an elimination to occur.
3.
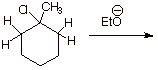
q Does proceed, to give the more stable alkene, which is 1-methylcyclohexene.
![]()
4. [2 pts] The following elimination does proceed. Draw the structure of the expected product.
Note that the conformation around the Ca-Cb bond is eclipsed. Predict whether the reaction is
fast or slow and explain why. [Hint: Consider the appropriate dihedral angle.]
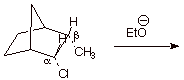
q The reaction is relatively slow, because there is no anti relationship possible between the beta hydrogen and the leaving group.
IV.Alcohols
A.
IUPAC Nomenclature
1. [2 pts each = 4 pts] Provide the correct and complete IUPAC names for the following alcohols.
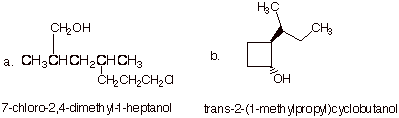
B. Conversion of Alcohols to
Ethers
1. [ 3 pts] Write the detailed
mechanism for the reaction shown below.
![]()
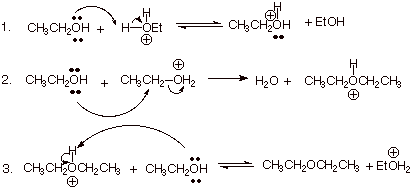
2. [ 2 pts] As a method for ether synthesis, is this a
good, general method for the preparation of ethers? Why or why not?
q No. It is limited to forming symmetrical ethers.
(Also to ethers having only two primary R groups).
C. Oxidation of Alcohols to
Carbonyl Compounds
1. [ 1 pt] A partial mechanism for the
oxidation of ethanol by chromic acid is shown below. What is the reaction
type for the last step of this
reaction?
![]()
q
An E2 elimination.
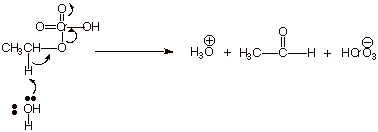
2. [2 pts] With the help provided by the DL/PC model for the TS of this reaction provided below, explain why this reaction proceeds so readily even when the only base available is the very weak base, water. In contrast, the elimination of an alkyl halide does not proceed at all using water as the base, but requires hydroxide anion. [Keep in mind that the leaving group is only an average leaving group. ] [Hint: Consider a character of this TS]
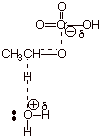
q
The TS has carbonyl double bond character, which is a highly stabilized double bond, much
more stabilized than the alkene character of the TS involved in the elimination of alkyl
halides.
D. [ 3 pts] Draw the structures of the products of the following reactions. The products must be products which can be obtained in good yield. If any special technique is required, specify the technique and the reasons that it must be used.
1.
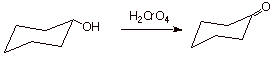
2.
![]()
q
This requires the distillation of the aldehyde away
from the aqueous oxidant as it as formed.
q Will accept the carboxylic acid as a product which can be obtained in good yield without using special precautions.
V.Ethers
A. IUPAC Nomenclature
1. [2 pts each = 4 pts] Provide correct and complete IUPAC names for the following compounds:
![]()
q a. ethyl propyl ether (or 1-ethoxypropane)
q b. 3-ethoxy-1-butanol
B. Williamson Ether Synthesis
1. [ 3 pts]Sketch two conceivable Williamson-type syntheses of the following ether, starting with any necessary alcohol and any necessary alkyl halide. Indicate which one of them does not work in any reasonable yield, and explain why not.
![]()
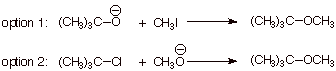
q Option 2 doesn’t work at all, because tertiary halides undergo E2 elimination in preference to substitution.
B. An Alternative Route to Di-tertiary-ethers.
1. [3 pts] The Williamson ether synthesis fails for ethers like di-tert-butyl ether (see below). Sketch an alternative, efficient synthesis for this ether.
![]()
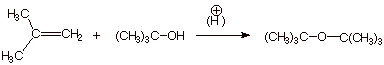
q Recall that addition of alcohols to alkenes gives ethers and is particularly good for alkenes which, when protonated, give tertiary carbocations. The tert-butyl carbocation then reacts with the alcohol as a nucleophile, to give the desired ether.
D. “Autoxidation” of Ethers.
1. [ 3 pts] Write the detailed mechanism for the air oxidation of diethyl ether to yield the indicated hydroperoxide product. You may use a general alkoxy radical (RO.) to show the initiation step.
![]()
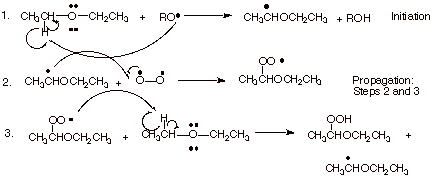
2. [1 pt] Explain why ethers are so readily oxidized under mild (room temperature) conditions, whereas air oxidation of alkanes requires higher temperatures.
q
The hydrogen alpha to the oxygen atom is easily
abstracted because the resulting radical is stabilized by three electron
bonding.
3. [2 pts] The reaction of the intermediate radicals with oxygen is essentially diffusion controlled (no activation energy). Explain why oxygen reacts so rapidly with the intermediate alkyl radicals.
q Dioxygen is a diradical. Its reactions with radicals are essentially radical coupling reactions.
E. Miscellaneous
1. [ 2 pts]Provide the name of the ether shown below, indicate which metal ion it is ably to coordinate especially powerfully, and explain why other metal ions, such as sodium or lithium, are not coordinated nearly so powerfully.
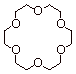
q
18-Crown-6.
q
Potassium ion.
q Potassium ion is just the right size to fit in the cavity formed by the macrocyclic polyether. Sodium is a little too small to give a good fit, and other metal ions are either too small or too large.
2. [2 pts] Provide the name of an analogous ether and the name of the metal ion with which it is able to selectively coordinate.
q 15-Crown-5 selectively complexes sodium ion. (12-Crown-4 complexes lithium ion.)
2. [ 2 pts] Indicate what advantage these ethers have as coordinators of metal ions in comparison with a simple ether like diethyl ether. Consider the possibility of using not just one molecule of diethyl ether, but multiple molecules. Specifically, why is one molecule of the macrocyclic ether in E.1 above better than six molecules of diethyl ether for coordinating metal ions.
q
When six ether molecules are incorporated into a
metal ion complex, this requires the immobilization and loss of the
translational entropy (freedom to move around independently) of six molecules.
When 18-crown-6 is used to supply all six coordinating oxygens, only one
molecule (the crown ether) is immobilized.
VI. Epoxides
A. Synthesis of Epoxides
1. [ 3 pts total] Predict the structures of the products of the following reactions. If no significant amount of reaction occurs, indicate that and explain why. (Note that hydroxide ion is used in catalytic amounts, and the reaction is carried out at room temperature).
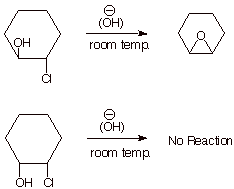
q
The displacement of chloride ion by an internal
alkoxide ion, like any SN2 reaction, requires a “backside
displacement” or inversion, in which the alkoxide function displaces the
chloride ion from the opposite face from which the chloride leaveds. This works
well in the first case, but in the second case the alkoxide is on the same face
as the chloride ion , so this reaction is impossible,
q
If the above explanation is given, but another
product is given (e.g., elimination or displacement by external hydroxide ion
to give a diol), full credit is given. But if the epoxide is specified as the
product, this will be seen as incorrect.
2. [ 2 pt] Indicate, via a synthetic sketch, how one of these molecules above (2-chlorocyclohexanols) could be prepared from cyclohexene. If only one of these halohydrins is obtained in your synthesis, give the reasons for this selectivity.
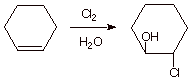
q
A single product, the trans product shown, is
obtained, because the intermediate epichloronium ion opens from the opposite
face upon reacting with water nucleophiles.
B. Cleavage of Ethers in Alkaline Media
1. [ 2 pts] Indicate whether the following reaction works. Explain the basis for your answer.
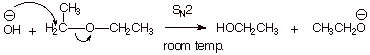
q
No,
the reaction fails, because the ethoxide anion is a poor
leaving group.
2. [2 pts] Indicate whether the following reaction works. If the reaction does work, explain why it works and draw the structure of the product obtained.
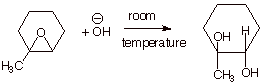
q The reaction does work, because the epoxide ring is quite highly strained, and although an alkoxide function is the leaving group, the loss of this group relieves the strain.
q Note that the correct structure of the product must have the hydroxyl groups trans to one another.
C. Cleavage of Epoxides in Acidic Media
1. [ 3 pts] Write the detailed mechanism for the cleavage of methyloxirane in acidic methanol.
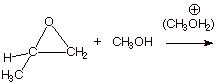
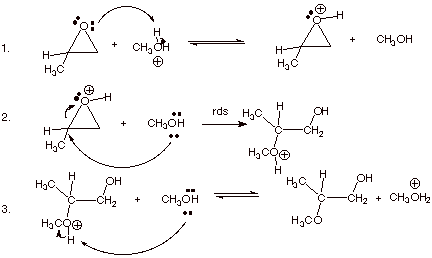
2. [ 3 pts] Provide a resonance theoretical treatment of the conjugate acid of this epoxide, summarize as a DL/PC structure. At which carbon is the positive charge largest? Explain in terms of resonance theory..
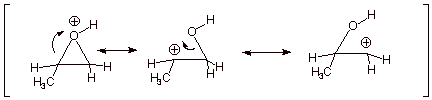
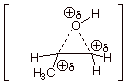
q
The largest positive charge on carbon is on the
secondary carbon (the carbon to which the methyl group is attached).
q This
is because the second structure above, which has positive charge on a secondary
carbon, is lower in energy than the third structure, which has positive charge
on a primary carbon. The resonance hybrid therefore more closely resembles the
second structure.
VII. Alkynes/
Synthesis
A. Nomenclature of Alkynes
1. [ 2 pts each = 4 pts] Draw the structure of one terminal alkyne and one non-terminal alkyne and provide the correctIUPAC name of each. Both of the alkynes must have more than four carbons atoms and at least one branch on the main chain.
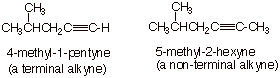
q
These are just two possible examples.
B. Acidity of Alkynes
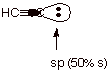
1. [ 4 pts] Cite the approximate pKa of ethyne (acetylene), and explain its high acidity in comparison to alkenes and alkanes. Your answer should include: (1) the general factor accounting for its enhanced acidity (2) the specific factor involved (3) an orbital depiction of the conjugate base of ethyne and (4) an explanation of how this factor distinguishes between the acidities of ethene, ethane, and ethyne.
![]()
q
Ca. 25
q
General Factor: Anion Stability
q
Specific Factor: Hybridization
q
The conjugate base of ethyne has the electron pair
in an sp AO, which is much lower in energy than the sp2 AO of the
conjugate base of ethene or the sp3 AO of the conjugate base of
ethane.
C. Synthesis of Alkynes
1. [ 3 pts] Sketch an efficient synthesis of 1-pentyne from 1-pentene. [Caution: Be specific and careful in your choice of the nature of the base used and the amount of it]
![]()
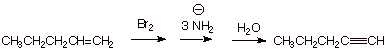
3. [ 2 pts]For which elimination step in your synthesis would ethoxide be sufficient? Which requires a stronger base? Why?
q
The first elimination, which gives a bromoalkene.
q The second elimination step, which gives the alkyne triple bond is the more difficult one, requiring a stronger base because the second double bond is essentially less stable than the first (or, better, the triple bond is less stable than the double bond).
4. [ 2 pts]How many moles of base are required in order to provide a good yield of the alkyne product? Explain why this particular number is required.
q
Three moles are required.
q
The first two are used to do the double elimination,
but the third mole is used to neutralize the terminal alkyne. This cannot be
avoided because the terminal alkyne hydrogen is so acidic. When it is
neutralized, if two moles are used, there will not be enough to complete the
second elimination of all of the substrate.
C. [3 pts] Sketch an efficient synthesis of the following target molecule, starting with ethyne as one key reactant and using any alkyl halide you may require.
![]()