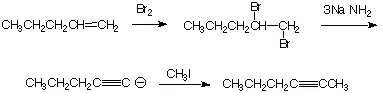CH 310M/318M
Final Exam
Fall, 2004
Prof. N. L. Bauld
__________________________________________ Name (Last,
First)
CH 310M/318M
Final Exam
Fall, 2005
Prof. N. L. Bauld
__________________________________________ Name (Last,
First)
Question |
Possible Points |
Page/Pts/Points Earned |
Score |
|
I. |
40 |
3/14/ 4/9/ 5/17/ |
|
|
II. |
28 |
6/12/ 7/10/ 8/6/ |
|
|
III. |
22 |
9/9/ 10/13/ |
|
|
IV. |
23 |
11/11/ 12/12/ |
|
|
V. |
16 |
13/7/ 14/9/ |
|
|
VI. |
12 |
15/12/ |
|
|
VII. |
9 |
16/9/ |
|
|
Totals |
150 |
|
|
I.Mechanisms of Nucleophilic Substitution Reactions
A. Nucleophilic Substitution Mechanisms
1. [5 pts] Write the detailed mechanism for the reaction shown below.
![]()
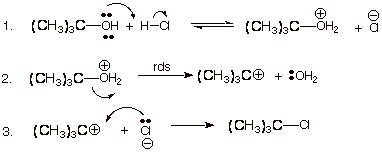
2.
[6 pts] The questions below
refer to the reaction immediately above in question A.1:
q
[2 pts] Symbolic
Classification of the Mechanism?
SN1
q
[1 pt] Significance of
the numeral?
Number of molecules
(molecularity) in the rds. Two here.
q
[2 pts] Reason the
reaction is classified as nucleophilic and not electrophilic?
o
From the point of
view of the organic species (the carbocation---it reacts with a nucleophile.
q
[1 pts] What species
acts as the electrophile?
o
The tert-butyl
carbocation.
o
We can also accept
HCl as the electrophile in the first step.
3. [3 pts] Does the
analogous reaction below proceed at a reasonable rate? Explain why or why not.
![]()
q
No, because the
hydroxide anion is a poor leaving group. A good leaving group is a weak base.
4. [5 pts]
Draw a reaction path diagram for the reaction above that is consistent with the
mechanism you wrote. On your diagram, label the coordinate axes and draw the structures of any intermediates involved on this reaction path.
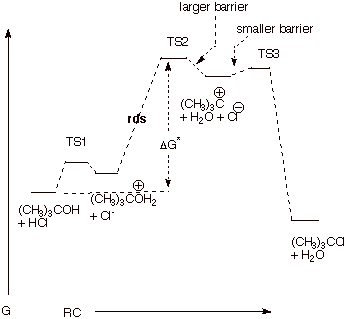
5. [2 pts] Indicate on your reaction path diagram
which step is the rds, and explain what feature(s) of your diagram are
consistent with this step as rigorously rate-determining.
q
The tert-butyl
carbocation virtually always goes on to products and never back to reactants
because the barrier to the back reaction is significantly larger than the small
barrier to the forward reaction which gives the products.
q
The second feature is
simply that the barrier to the final reaction step is very small, so that the
concentration of the carbocation never builds up; i.e., it goes rapidly on to
product.
6. [2 pts] Illustrate on your diagram the activation
free energy for the reaction.
q
Shown on the diagram.
We will also accept the (not quite correct) answer which shows the
activation energy as that of the second step alone (i.e., the energy difference
between the conjugate acid of tert-butyl alcohol + chloride ion and the energy
of the TS for the rds.
7. [8 pts] Provide a resonance theoretical treatment of the transition state for the rate determining step of the reaction, summarize as a dotted line/partial charge structure and characterize the TS model.
q
Resonance Treatment [3
pts]:
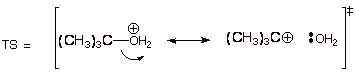
q
DL/PC [2 pts]:
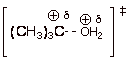
q
Characterization of TS
Model [3 pts]:
o
Tertiary carbocation
character
5. [4
pts] State
the Hammond Principle in its most succinct form, apply it to the rds of the
reaction to further refine the TS characterization.
q
The TS of a reaction
step more closely resembles the higher energy partner (reactant or product of
that step).
q
Since the rds is
strongly endothermic, the TS more closely resembles the product of the step,
which is the tert-butyl carbocation.
q
Thus, the TS has
extensive tertiary carbocation character.
5.
[2 pts] Apply the Hammond Principle to the last step of the reaction mechanism
and use this to describe the character of the TS for this last step of the
reaction.
q
The last step is
strongly exothermic, so the TS more closely resembles the reactant of that
step.
q
Therefore, the TS
again closely resembles the tert-butyl carbocation, and the TS of the last step
again has extensive carbocation character.
5. [3
pts] Consider the analogous reaction of
2-chloropropane with water to give 2-propanol. What mechanism (symbolic
mechanistic classification) is operative in this reaction? Explain, in detail,
what considerations allow us to make this prediction. Consider the primary,
secondary, tertiary classification of the carbon undergoing substitution and
the strength of the reacting nucleophile.
![]()
q
Secondary systems are
not especially good at either mechanism.
q
The deciding factor is
the strength of the nucleophile. Strong nucleophiles favor the SN2
mechanism.
q
Chloride ion is a
strong nucleophile, so the mechanism is SN2.
II. More On Nucleophilic Substitutions
A. Consider the nucleophilic substitution reaction given in the equation below:
![]()
1. [2
pts] Based upon the structure of the alkyl halide involved in this reaction,
you should be able to predict the kind of mechanism involved. What is the
symbolic mechanistic designation for this reaction?
q
SN2
2. [2]
Considering only the number of steps involved in the reaction, what is the
appropriate term which applies to a reaction having this number of steps?
q
Concerted (because
there is just one step).
3. [2 pts] Write down the form of the kinetic rate law for
this reaction, and define the term “rate constant”.
q
The rate constant, k,
is the rate of a reaction at unit concentrations of all reactants. (It
represents the intrinsic rate of the reaction, independent of concentration).
q
The rate law is: rate
= k[CH3Cl][OH-‑]
4. [6 pts] Provide a complete resonance
theoretical treatment (derive) for
the rds of this reaction, summarize
as a DL/PC structure, characterize
the TS, and indicate what kind of structural effect this character is especially sensitive to.
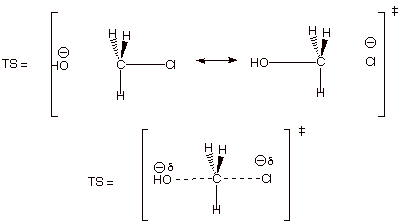
q The TS has pentacovalent (or pentacoordinate)
carbon character.
q It is sensitive to steric effects!
B. Solvent Effects
1.
[3 pts] Using the DL/PC model of the TS for the rds of the reaction of
chloromethane with the hydroxide anion (IIA. above), provide a prediction of
the effect upon the reaction rate of this reaction when the solvent polarity is
increased. Be specific, and explain your reasoning.
q
Ions are stabilized
by solvation by polar solvents. A polar solvent will stabilize the reactant
hydroxide anion.
q
A polar solvent will
also stabilize the TS anion. The net result is that there is no large effect of
solvent polarity upon the rate of this reaction.
q
However, smaller ions
are more efficiently solvated and stabilized than larger ions, so the hydroxide
ion is somewhat more stabilized than the TS anion. Thus the rate is modestly
diminished by increasing solvent polarity.
2.
[7 pts] Finally, suppose that the solvent in that same reaction is changed from
a
hydroxylic solvent (like methanol) to dimethyl formamide or
dimethylsulfoxide.
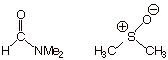
- [2 pts] Provide the generic name which describes
the kind of solvent that dimethylsulfoxide or dimethylformamide is:
q
Dipolar aprotic.
- [3 pts] Explain, with the assistance of a
depiction, why the rate of reaction with hydroxide ion is greatly
accelerated in these latter solvents.
q
A hydroxylic solvent
strongly hydrogen bonds to the nucleophile anion, making it less reactive.
q
In an aprotic solvent,
there is no hydrogen bonding, so the anion is much more reactive.
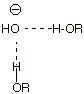
- [2 pts]
Finally, explain, again with the assistance of a depiction, why
ionic substances such as sodium hydroxide, are so soluble in
dimethylsulfoxide.
q
The cation is
strongly stabilized by solvation with the highly negatively charge oxygen end
of the S-O dipole, thus providing the thermodynamic driving force for
dissolution of the salt in DMSO.
q
But the anion is not
highly solvated (the S is positively charged, but has sizeable groups around
it, preventing very close association with the anion).
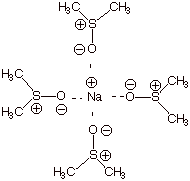
C. Stereochemistry.
1.
[4 pts] Sketch the mechanism of the reaction [consider the type of
halide and the strength of the nucleophile in deciding which mechanism is
operative] shown below so as to illustrate the stereochemical outcome of the
reaction. Draw the structure(s) of the product(s) and provide the term which describes this stereochemical outcome. [Again,
the “squiggly line indicates an unspecified stereochemistry, which is for
you to specify.]
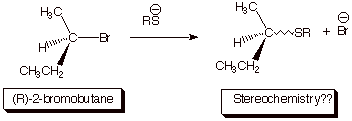
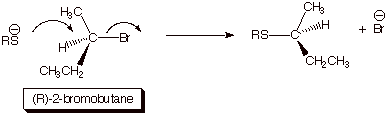
q
The halide is secondary, so it could go either way, but the sulfur
nucleophile is strong, thus favoring an SN2 mechanism.
q
Sulfur nucleophiles are
strong because of the polarization effect (soft electrons) around a heavier
atom.
q
Consequently, the
reaction occurs with 100% inversion
of stereochemistry.
D. Leaving Group Ability
1.
[ 2 pts] Hydrogen bromide (HBr) is a significantly stronger acid than hydrogen
chloride (HCl). Using this observation, predict whether bromide anion or
chloride anion is a better leaving group in nucleophilic substitution
reactions. Explain the basis for your conclusions.
q
The bromide ion is a
better leaving group than the chloride ion.
q
A good leaving group
is a weak base. Bromide ion is a weaker base than chloride ion, since the
conjugate acid of bromide ion is a stronger acid than the conjugate acid of
HCl.
III.Elimination Reactions;
Competition with SN Reactions
A. Mechanism of Elimination
Reactions
1. [5 pts] Use resonance theory to derive a TS model
for the elimination reaction shown below, summarize as a DL/PC structure, and
characterize the resulting TS model.

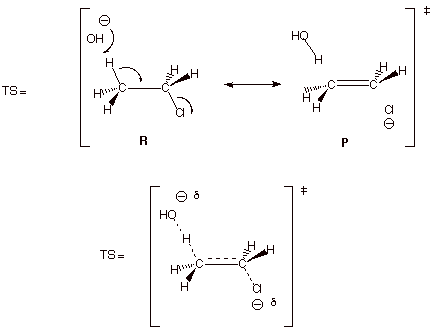
q The TS has alkene character.
q
2. [1 pt]
Assuming that this is a concerted reaction, what is the symbolic
mechanistic designation for the reaction mechanism?
q E2
3.
[2 pts] Is this reaction base catalyzed? Why or why not? What is the
appropriate term to describe the role of the base in this reaction?
q No. the base is consumed in the reaction, and
converted to water. A stoichiometric amount of base is needed for the reaction.
q Base-promoted.
4.
[1 pts] What term is used to describe the role of chloride ion in this
reaction?
q It is a Leaving Group.
B. Selectivity in Systems in
Which There are Two Non-Equivalent Beta Hydrogens
1. [5 pts] Provide the structures and names of the products of the following reaction. Designate the alpha and the two distinct beta positions of the reactant and indicate which product arises from the removal of which beta hydrogen, and which product is the major product.
![]()
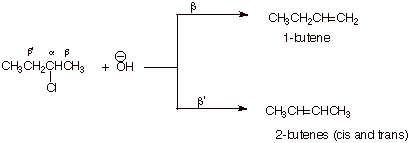
q
The 2-butenes are the major products.
2.
[4 pts] Use
the Method of Competing Transition States to compare the TS’s for the
reactions leading to these products. Apply the respective characters of these
transition states to explain the predominance of the product which is observed
to be major.
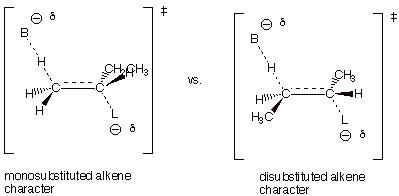
q
The TS on the left,
which leads to 1-butene, has monosubstituted alkene character, while that on
the right, which leads to the 2-butenes, has disubstituted alkene character.
q
The 2-butenes are the
major products since the more highly substituted alkene character is favored
(or alkyl groups stab ilize alkenes and alkene character).
3.
[4 pts] The selectivity in these eliminations is seen to be relatively low,
i.e., both products are formed in very substantial amounts. Provide two
distinct reasons for this lack of selectivity. One of these reasons should
involve an application of the Hammond Principle.
o
Since the reaction
“goes” (i.e., it is spontaneous), it is exothermic. Therefore the
TS at least somewhat more closely resembles the reactants than the products of
this step. The product is the alkene, so the TS has only modest alkene
character.
o
The full amount of
the difference in stability between a monosubstitued and a disubstituted alkene
is only 2.7 kcal/mol, so even if the TS had extensive alkene character, the
selectivity would only be moderate.
IV.Alcohols
A.
IUPAC Nomenclature
1. [3 pts] Provide the correct and complete IUPAC name for the following alcohol. Indicate the numbering system that you are using.
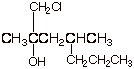
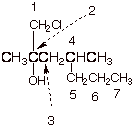
q 1-chloro-2,4-dimethyl-2-heptanol
q Note that the numbering system begins at the end of the parent chain closest to the carbon containing the hydroxyl group. C comes before m in the alphabetic listing.
B. Conversion of Alcohols to
Ethers
1. [3 pts] Sketch two different synthetic routes by which
the following ether can be efficiently synthesized,using an alkyl halide and an alcohol as the starting materials.
![]()
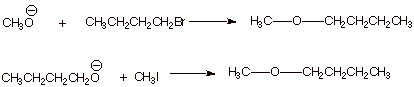
q The
alkoxide can be prepared by reaction of the alcohol with sodium metal.
2. [1 pts] What is the appropriate name by which this
kind of ether synthesis is commonly known (its inventor)?
q Williamson synthesis
3. [2 pts] What is the mechanistic symbol for the key
reaction involved in this ether synthesis? Explain why this particular
mechanism is operative.
q An SN2 mechanism.
q Mainly because SN1 mechanisms do not
generally operative in methyl or primary systems.
4. [2 pts] Describe the limitations of this synthetic
method, and ndicate specifically what other reaction can compete with this
ether synthesis.
q Can only be done for methyl or primary systems.
q This is because it uses a strong base, and a strong
base will cause E2 elimination with a secondary or tertiary system.
C. Oxidation of Alcohols to
Carbonyl Compounds
1. A partial mechanism for the
oxidation of ethanol by chromic acid is shown below.
![]()
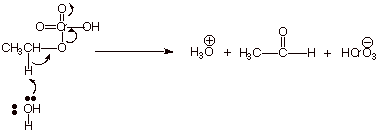
a. [6 pts] Use resonance theory to derive a TS model for this last step and summarize it as a DL/PC model.
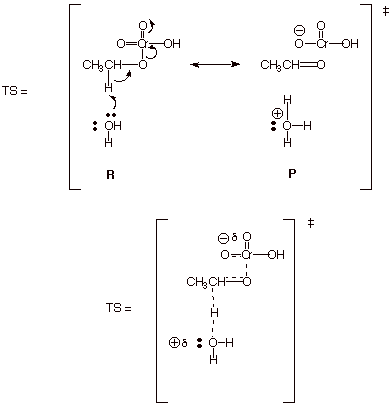
b. [4 pts] Characterize this model with respect to the organic part of the TS, and why this specific character is of great importance in providing for a relatively rapid reaction.
q
It has carbonyl double bond character.
q The carbonyl double bond is an especially highly stabilized double bond, so this provides stabilization for the TS.
c. [2 pts] What kind of mechanism is involved in this reaction (symbol).
q E2
V.Ethers
A. IUPAC Nomenclature
1. [2 pts each = 4 pts] Provide correct and complete IUPAC names for the following compounds:
![]()
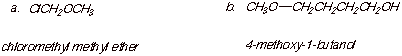
B. “Autoxidation” of Ethers
1. The overall reaction for the air oxidation of diethyl ether to yield an explosive hydroperoxide product is shown below, following by the detailed mechanism for this reaction.
![]()
Mechanism:
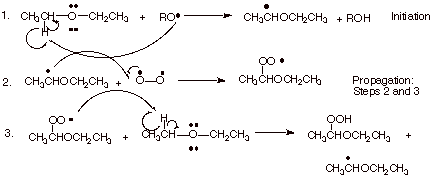
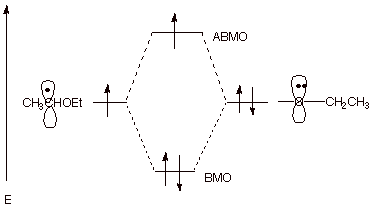
a. [3 pts]Indicate the name of the kind of special stabilization the organic radical formed in the first step has and illustrate this by means of an MO energy level diagram.
q
This radical has 3e bonding stabilization resulting
from the overlap of the 2p AO at the radical site with the filled 2p AO on
oxygen.
b. [2 pts] Also, indicate the name of the kind of special stabilization which the radical formed in the second step (a peroxy radical) has. Explain the basis for this stabilization of peroxy radicals.
q
The peroxy radical also has 3 electron bonding
stabilization, resulting form the overlap of the 2p orbital at the oxygen
radical center with the filled 2p AO on the attached oxygen atom.
c. [2 pts] Explain, in terms of the structure of dioxygen, why the second step occurs at the highest possible rate for any reaction in solution (the diffusion controlled rate).
q
Dioxygen is a diradical, so the reaction with an
organic radical is essentially a radical-radical coupling, which requires
little or no activation energy.
E. Miscellaneous
1. [ 3 pts]Provide the name of the ether shown below, indicate which metal ion it is ably to coordinate especially powerfully, and explain why other metal ions, such as sodium or lithium, are not coordinated nearly so powerfully.
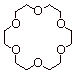
q
This is the 18-crown-6 ether.
q
It especially coordinates the potassium ion.
q
The cavity size is just right for the larger
potassium ion, but too large for the sodium or lithium ions to simultaneously
interact with all six oxygen atoms.
2. [2 pts] Provide the name of an analogous ether which is able to effectively coordinate lithium ions.
q 12-crown-4VI. Epoxides
A. Cleavage of Ethers in Alkaline Media
1. Consider the following hypothetical reaction:
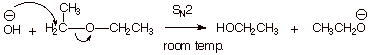
a. [ 2 pts] What type of reaction would this be (symbol) if it occurred as indicated.
q SN2
b. [ 2 pts] What is the leaving group in this hypothetical reaction.
q
The ethoxide ion.
c. [ 2 pts] The reaction doesn’t occur because this leaving group is a very poor leaving group. Explain on the basis of a general principle how we would have known that this is a poor leaving group.
q
Good leaving groups are weak bases.
d. [ 2 pts] Now, suppose that we make the solution acidic, instead of basic. In terms of carrying out the desired cleavage reaction of this ether to an alcohol, what are the “pro’s and cons” of this substitution (what would be better about the use of acid, and what would be worse?)?
q
The leaving group would then be ethanol, which is
indeed a weak base and a good leaving group. So this would be better.
q
But, in acidic solution a strong base like hydroxide
ion would not be available. The best base available would be water, and that is
a very weak base. This would be worse.
C. Cleavage of Epoxides in Acidic Media
1. [4 pts] Provide a complete resonance theoretical treatment of the conjugate acid of the epoxide of propene (methyloxirane), and summarize as a DL/PC structure. At which carbon is the positive charge largest? Explain in terms of resonance theory..
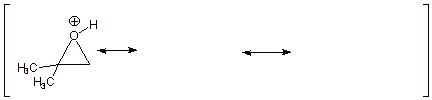
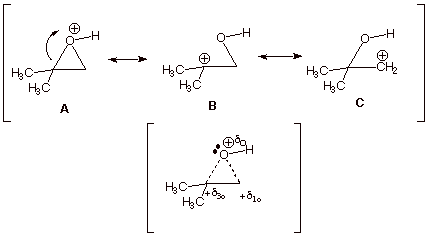
q Structures B and C show carbocation character on both epoxide carbon atoms, but structure B is of lower energy than structure C, because in B the carbocation center is tertiary and in C it is primary. Therefore B contributes more than C to the resonance hybrid, and there is more carbocation character at the tertiary carbon.
VII.
Alkynes/ Synthesis
A. Nomenclature of Alkynes
1. [ 2 pts] Draw the structure of a terminal alkyne and provide its correct IUPAC name.. The alkyne must have more than four carbons atoms and at least one branch on the main chain.
![]()
q 4-methyl-1-pentyne
B. Acidity of Alkynes
1. [4 pts] Cite the approximate pKa of ethyne (acetylene), and explain its high acidity in comparison to alkenes and alkanes. Your answer should include: (1) the general factor accounting for its enhanced acidity (2) the specific factor involved (3) an orbital depiction of the conjugate base of ethyne and (4) an explanation of how this factor distinguishes between the acidities of ethene, ethane, and ethyne.
![]()
q
The
pKa of ethyne is ca. 21.
q
The general factor which accounts for the unusually
high acidity of ethyne is anion stability.
q
The specific factor which accounts for anion
stability is hybridization. In
the ethyne conjugate base, the unshared pair is in an sp AO, which is the
lowest energy of the hybrid AO’s. In the case of the conjugate base of
ethene, the unshared pair is in an sp2 AO and for ethane an sp3
AO. The order of stability parallels the s content, going from 50% for
the alkyne conjugate base, to 25% for the ethane conjugate base.
![]()
C. Synthesis of Alkynes
1. [3 pts] Sketch an efficient synthesis of the the alkyne shown below, starting with 1-pentene. [Caution: Be specific and careful in your choice of the nature of the base used and the amount of it]
![]()