CH 318M (Majors)
Final Exam
Fall, 2004
Prof. N. L. Bauld
__________________________________________ Name (Last,
First)
CH 318M (Majors)
Final Exam
Fall, 2004
Prof. N. L. Bauld
__________________________________________ Name (Last,
First)
Question |
Possible Points |
Page/Pts/Points Earned |
Score |
|
I. |
29 |
3/13/ 4/16/ |
|
|
II. |
24 |
5/13/ 6/11/ |
|
|
III. |
34 |
7/15/ 8/12/ 9/7 |
|
|
IV. |
15 |
10/12/ 11/3/ |
|
|
V. |
17 |
12/9/ 13/8/ |
|
|
VI. |
12 |
14/12/ |
|
|
VII. |
19 |
15/9/ 16/10 |
|
|
Totals |
150 |
|
|
I.Mechanisms of Nucleophilic Substitution Reactions
A. Nucleophilic Substitution Mechanisms
1. [5 pts] A certain substrate which conforms to the
general structure R-L (see below) reacts with a certain nucleophile of
generalized structure Nu-‑. The rate of the reaction in this
specific case was found to be independent of the concentration of the salt Na+ Nu- which
contains the nucleophilic anion. Write the detailed mechanism for this reaction
and the mechanistic symbol. Explain why the rate of the reaction is independent
of the concentration of the nucleophile.
![]()
q
The symbol is SN1.
q
The rate is
independent of the concentration of the nucleophile because the nucleophile is
not present in the rate-determining step, which is the heterolysis of the R-L
bond.
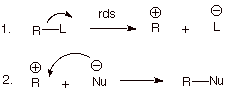
2. [5 pts] Draw a reaction
path diagram for the reaction and indicate on this diagram (1)the labels of the
coordinate axes (2) the structures
of species which are free energy minima.and (3) the activation free energy for
the reaction.
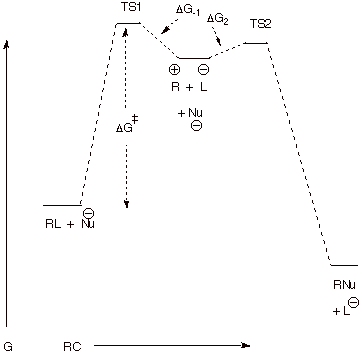
3. [3 pts] Indicate on your
reaction path diagram, and by explanation in the space below, the requirements
(two) for a given step of a reaction to be rigorously rate-determining.
q
The intermediate must
not return to reactants, but always go on to products. This is fulfilled by
having the activiation energy for the reversal of the first step be
substantially larger than that for the forward second step.
q
The intermediate must
not build up substantially in concentration. This is fulfilled by the
circumstance that the activation energy for the second step be very small
(conversion to the product is very fast).
4. [3 pts] If the rds is
partially reversible, is that step still classified as rigorously rate-determining?
Why or why not? What would be the appropriate term used to describe this step
if it were significantly but only modestly reversible?
5. [6 pts] Employ resonance
theory to derive a TS model for the rate-determining step of the reaction,
summarize as a DL/PC, and characterize this TS model. Extend your
characterization by invoking the Hammond Principle.
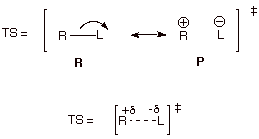
q
The TS has carbocation
character.
q
Since the step is
endothermic, the TS has extensive carbocation character.
6. [5 pts] For the reactions of
various R-L, where the nature of R is varied as shown below, but the same
mechanism is operative as in the first question (A.1), indicate the expected
sequence of rates (1 = fastest). Explain your answer by using the Method of
Competing Transition States (i.e., provide a TS model and characterization for
each of these R groups).
![]()
![]()
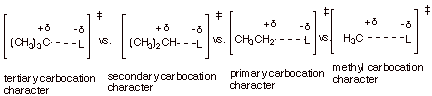
q
Tertiary carbocation
character is the most favorable, followed by secondary, then primary, then
methyl as the least favorable.
7. [2 pts] Is the difference in rates between the
various R groups (e.g., tert-butyl
and isopropyl) expected to be large or small? Explain, giving two reasons for
your answer.
q
Large. First because
the difference in stability between a tertiary and a secondary carbocation is
large (about 20 kcal/mol).
q Second, because the TS has extensive carbocation character. A large fraction of the total carbocation stabilizing effect is felt in the TS.
II. More On Nucleophilic Substitutions
A. Consider the nucleophilic substitution reaction given in the equation below:
![]()
1. [2
pts] Based upon the structure of the alkyl halide involved in this reaction,
you should be able to predict the kind of mechanism involved. What is the
symbolic mechanistic designation for this reaction?
q
SN2
2. [2]
Considering only the number of steps involved in the reaction, what is the
appropriate term which applies to a reaction having this number of steps?
q
Concerted
3. [3 pts] Write down the form of the kinetic rate law for
this reaction, and define the term “rate constant”.
q
Rate = k [CH3CH2Cl][OH-‑]
q
The rate constant, k,
is the rate of the reaction at unit concentrations of all reactants (it represents the intrinsic rate of the reaction).
4. [6 pts] Provide a complete resonance
theoretical treatment (derive) for
the rds of this reaction, summarize
as a DL/PC structure, characterize
the TS, and indicate what kind of structural effect this character is especially sensitive to.
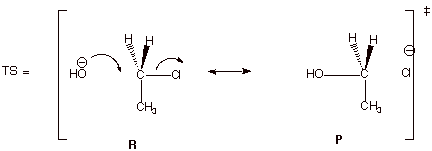
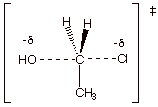
q
The TS has
pentacovalent carbon character
q
It is especially
sensitive to steric effects.
B. Solvent Effects
1. [3 pts] Using the DL/PC model of the TS for the rds of
the reaction of chloroethane with the hydroxide anion (IIA. above), provide a
prediction of the effect upon the reaction rate of this reaction when the
solvent polarity is increased. Be specific, and explain your reasoning.
q
Increased solvent
polarity has the effect of modestly (or slightly) slowing down the reaction.
q
This is because the
reactant and product side both are anionic, and both will be stabilized by
solvation. For this reason the effect upon the rate is small.
q
The rate is decreased
because solvation is more effective for smaller ions, and the hydroxide anion
is smaller than the TS anion (which contains the hydroxide ion).
C. Stereochemistry of SN2
Reactions.
1. [5 pts] SN2 reactions are known to
occur exclusively via a backside
(invertive) displacement. Using an appropriate illustration to support your
answer, provide an explanation for this powerful preference for inversion over
retention (frontside displacement) based upon MO theory (specifically frontier
MO theory or HOMO/LUMO theory or Filled Orbital/Vacant Orbital Interactions).
Explain why the frontside attack mode would be highly unfavored and why the
factor which makes fronside attack unfavorable is not operative for a backside
attack.
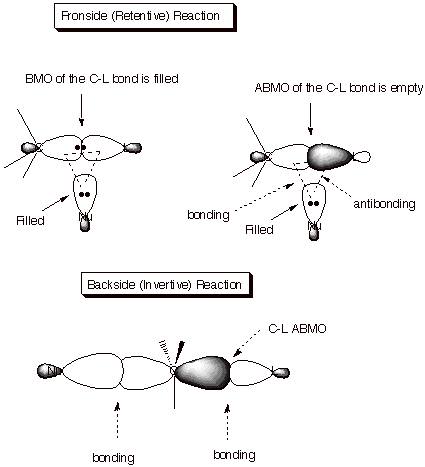
q
Recall that strong
bonding requires two electrons. The interaction between two filled orbitals
gives four electrons, which results in no bonding. Therefore the interaction
between the filled nucleophile orbital and the BMO corresponding to the C-L
sigma bond provides no bonding interaction.
q
But the interaction
between the filled nucleophile orbital and the vacant antibonding orbital
corresponding to the C-L sigma bond could potentially give rise to bonding.
Unfortunately, positive overlap of
the nucleophile with the carbon AO is at least partially or wholly negated by
the antibonding interaction between the nucleophile orbital and the orbital on
the leaving group, as shown in the diagram.
q
On the other hand the
interaction between the filled nucleophile orbital and the antibonding orbital
of the C-L bond (shown with sp2 hybridization) can be bonding
without the interference of the negative overlap with the leaving group
orbital, because the distance between Nu and L is too large for any effective
interaction.
2. [3 pts] Provide another reason for the preference for
inversion based upon the shape of the LUMO of the C-L bond. Again, provide an
appropriate illustration to support your answer and explain why the LUMO has
this shape.
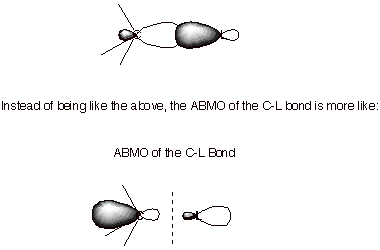
q
This is because
interference between the positive and negative lobes drives electron density
away from the antibonding region between the nuclei and more toward the back
lobe.
q
For this reason,
better overlap with the nucleophile is available from the back lobe on carbon
than on its front lobe.
III.Elimination Reactions;
Competition with SN Reactions
A. Mechanism of Elimination
Reactions
1 [7 pts] Use resonance theory to derive a TS model
for the elimination reaction shown below, summarize as a DL/PC structure, and
characterize the resulting TS model carefully and completely. Your resonance
treatment should include all three
good resonance structures.
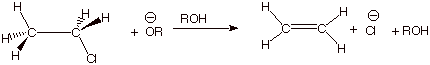
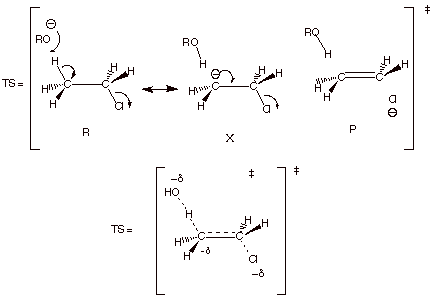
q The TS has alkene character.
q It also has some carbanion character at the beta
carbon.
2. [3 pts] Which character of the TS is dominant when
chloride ion is the leaving group? What is the proper name which is associated
with eliminations of this kind?
q
Alkene Character.
q
Saytzeff or Zaitsev
elimination.
3. [2 pt]
Assuming that this is a concerted reaction, what is the symbolic
mechanistic designation for the reaction mechanism?
q
E2
4.
[3 pts] Is this reaction base catalyzed? Why or why not? What is the
appropriate term to describe the role of the base in this reaction?
q No. The base is consumed stoichiometrically in the
reaction.
q Base-promoted.
B. Elimination vs Substitution
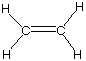
1. [2 pts] In the reaction above between chloroethane and
an alkoxide anion, another product can be obtained. Write the structure of that
product.
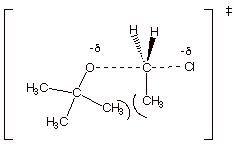
2. [5 pts] When ethoxide ion (R = Et) is used as the base
one of these two possible products is the major one and when tert-butoxide is
used as the base (R = tert-butyl) the other is the predominant product.
Indicate which base gives which product and explain in detail, with supporting
illustrations, the basis for this disparate behaviour.
q
With ethoxide the main
product is the SN2 product, because SN2 reactions at
primary carbon atoms are fast.
q
With the tert-butoxide ion the main product is ethene. This is
partly because this later base is a stronger base (more than 100 fold) than
ethoxide.
q
But especially because
the the tert-butoxide ion is a
somewhat hindered base, which makes the SN2 TS more congested and
less favorable.
B. Selectivity in Systems in
Which There are Two Non-Equivalent Beta Hydrogens
1. [5 pts] Provide the structures and names of the products of the following reaction. Designate the alpha and the two distinct beta positions of the reactant and indicate which product arises from the removal of which beta hydrogen, and which product is the major product.
![]()
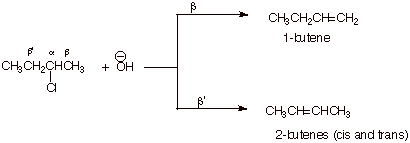
q
The 2-butenes are the major products.
2.
[4 pts] Use
the Method of Competing Transition States to compare the TS’s for the
reactions leading to these products. Apply the respective characters of these
transition states to explain the predominance of the product which is observed
to be major.
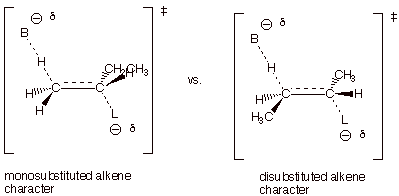
q
The TS on the left,
which leads to 1-butene, has monosubstituted alkene character, while that on
the right, which leads to the 2-butenes, has disubstituted alkene character.
q
The 2-butenes are the
major products since the more highly substituted alkene character is favored
(or alkyl groups stab ilize alkenes and alkene character).
3. [3 pts] The selectivity in these eliminations is seen
to be relatively low, i.e., both products are formed in very substantial
amounts. Provide two distinct reasons for this lack of selectivity. One of
these reasons should involve an application of the Hammond Principle.
o
Since the reaction
“goes” (i.e., it is spontaneous), it is exothermic. Therefore the
TS at least somewhat more closely resembles the reactants than the products of
this step. The product is the alkene, so the TS has only modest alkene
character.
o
The full amount of
the difference in stability between a monosubstitued and a disubstituted alkene
is only 2.7 kcal/mol, so even if the TS had extensive alkene character, the
selectivity would only be moderate.
IV.Alcohols
A.
IUPAC Nomenclature
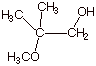
1. [3 pts] Provide the correct and complete IUPAC name for the following alcohol. Indicate the numbering system that you are using.
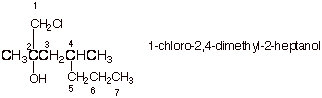
B. Conversion of Alcohols to
Ethers
1. [4 pts] Use Retrosynthetic Analysis to devise two
different synthetic routes by which the following ether can be efficiently
synthesized,using an alkyl halide
and an alcohol as the starting
materials. Sketch the synthetic routes.
![]()
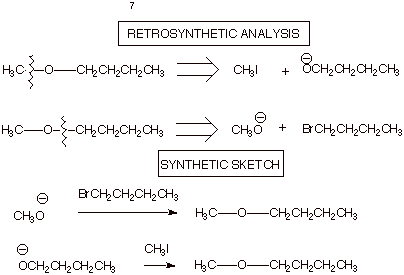
q Note
to Graders: Give one extra point if the preparation of the alkoxide from the
alcohol plus sodium is shown.
2. [1 pts] What is the appropriate name by which this
kind of ether synthesis is commonly known (its inventor)?
q Williamson Synthesis
3. [2 pts] What is the mechanistic symbol for the key
reaction involved in this ether synthesis? Explain why this particular
mechanism is operative.
q SN2
4. [2 pts] Describe the limitations of this synthetic
method, and indicate specifically what other reaction type can compete with
this type of ether synthesis.
q Only applies to methyl and primary halides.
q Competition is with E2 elimination, especially for
secondary and tertiary halides.
B. pKa’s of Alcohols.
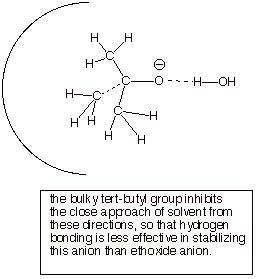
1. [3 pts] Explain why the pKa of tert-butyl alcohol is considerably higher than that of primary and secondary alcohols. Provide the specific name of the effect which makes tert-butyl alcohol a weaker acid, as well as a pictorial illustration of the effect.
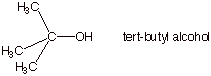
q
The tert-butoxide anion is less efficiently solvated
than an unhindered alkoxide, so the former is less stable. Decreased anion
stability engenders decreased acidity.
q The effect is termed “steric hindrance to solvation”.
V.Ethers
A. IUPAC Nomenclature
1. [3 pts each = 6 pts] Provide correct and complete IUPAC names for the following compounds:
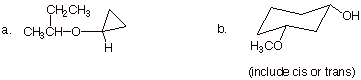
q
a. 2-cyclopropoxybutane (preferred) or cyclopropyl
2-butyl ether (okay)
q
b. cis-3-methoxycyclohexanol
B. “Autoxidation” of Ethers
1. The overall reaction for the air oxidation of diethyl ether to yield an explosive hydroperoxide product is shown below, following by the detailed mechanism for this reaction.
![]()
Mechanism:
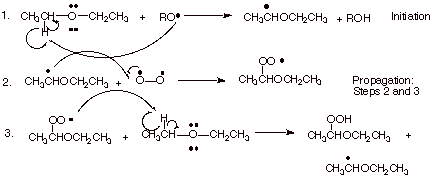
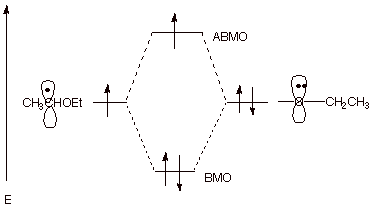
a. [3 pts]Indicate the name of the kind of special stabilization the organic radical formed in the first step has and illustrate this by means of an MO energy level diagram.
q
Three electron bonding.
b. [2 pts] The bond dissociation energy, D, of a peroxidic O-H bond is much less than than of an alcohol or water O-H bond. Provide an explanation for the relative weakness of the peroxide O-H bond. .
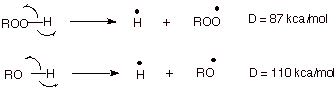
q
The peroxy radical also has three electron bonding.
The radical site is on oxygen, and the other oxygen supplies the unshared
electron pair.
F. Cleavage of Ethers
1. [4 pts] Although ethers are, relatively speaking, a rather unreactive class of organic compounds, they are effectively cleaved by hydrogen bromide. Write the detailed mechanism for the cleavage of the ether depicted below to form an alkyl bromide and an alcohol. Explain why the ether cleaves in the specific direction that it does cleave.
![]()
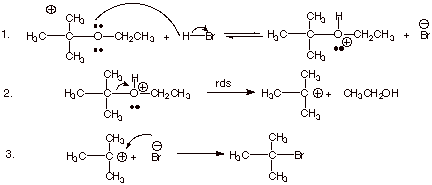
q
The cleavage could go by an SN1 mechanism
as shown, because the tert-butl carbocation is relatively stable, but it could
go by an SN2 reaction on the primary carbon to cleave off the ethyl
group as ethyl bromide.
q The cleavage occurs as it does because a unimolecular reaction is inherently faster than a biomolecular reaction.
2. [2 pts] Explain why HBr is much more effective in cleaving ethers than is HCl. What does this imply about the reactivity of HI? Why?
q
The reaction is generally (except in the case of
tertiary ethers) an SN2 reaction, so the stronger the nucleophile
the faster the rate.
q
Bromide ion is a much stronger nucleophile than
chloride ion (the polarization or soft electron effect).
q Reaction is even faster with HI.
VI. Epoxides
A. Cleavage of Epoxides in Acidic Media
1. [5 pts] Provide a complete resonance theoretical treatment of the conjugate acid of the epoxide of propene (methyloxirane), and summarize as a DL/PC structure. At which carbon is the carbocation character largest? Explain in terms of a simple axiom of resonance theory..
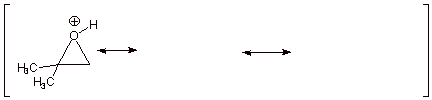
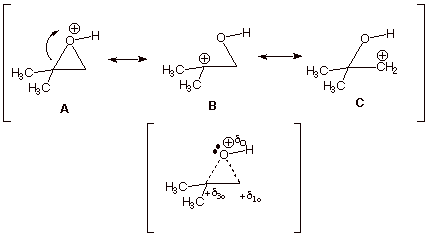
q Structures B and C show carbocation character on both epoxide carbon atoms, but structure B is of lower energy than structure C, because in B the carbocation center is tertiary and in C it is primary. Therefore B contributes more than C to the resonance hybrid, and there is more carbocation character at the tertiary carbon..
2. [2 pts] Write the structure(s) or the product(s) obtained when this conjugate acid reacts with methanol.
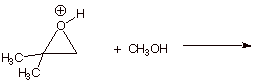
3. [3 pts] If there is any selectivity in this reaction, what type of selectivity is involved. Explain the theoretical basis for the particular kind of selectivity observed here.
q
Regioselectivity
q
The nucleophile tends to react at the position which
has the greatest amount of carbocatioin character (positive charge
accumulation).
q Extra 2 points: If it is mentioned that this reaction is strongly exothermic, so the TS resembles the reactant of this step, which is the oxonium ion (which has extensive carbocation character and relatively little product character).
q Therefore the covalent bond to the nucleophile is weak and long in the TS. Therefore, steric effects are small, and charge effects are large.
5. [2 pts] In what respect is the site of reactivity of the conjugate acid somewhat surprising?
q The nucleophile reacts at a tertiary carbon, which is highly hindered, instead of at a much less hindered primary carbon.
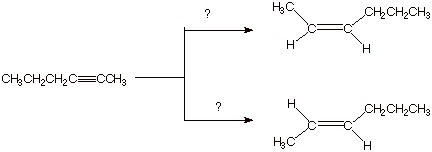
VII. Alkynes Synthesis
A. Nomenclature of Alkynes
1. [ 2 pts] Draw the structure of a non-terminal (internal) alkyne and provide its correct IUPAC name.. The alkyne must have more than five carbons atoms and one alkyl and one halogen substituent.
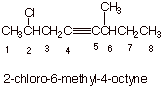
q
As just one example.
B. Acidity of Alkynes
1. [4 pts] Cite the approximate pKa of ethyne (acetylene), and describe its acidity in comparison to alkenes and alkanes. Your answer should include: (1) the general factor accounting for its differential acidity (2) the specific factor involved (3) an orbital depiction of the conjugate base of ethyne and (4) an explanation of how this factor distinguishes between the acidities of ethene, ethane, and ethyne.
![]()
q
The pKa of ethyne is ca. 21.
q
The general factor which accounts for the unusually
high acidity of ethyne is anion stability.
q
The specific factor which accounts for anion
stability is hybridization. In the ethyne conjugate base, the unshared pair is
in an sp AO, which is the lowest energy of the hybrid AO’s. In the case
of the conjugate base of ethene, the unshared pair is in an sp2 AO
and for ethane an sp3 AO. The order of stability parallels the s
content, going from 50% for the alkyne conjugate base, to 25% for the ethane
conjugate base.
![]()
2. [3 pts] Consider the bond dissociation energies of the C-H bonds shown below. Which C-H bond has the larger D? Explain why.
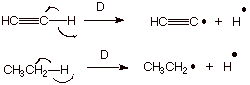
q
The ethyne C-H bond is stronger, i.e., has the
larger D.
q
The C-H bond of ethyne is formed using an sp
orbital, whereas that in ethane is formed using an sp3 AO.
q
Since the sp orbital is much lower in energy, the BMO
formed from it is also of much lower energy.
3. [3 pts] Indicate what kind of bond cleavage is occurring in B1 and B2 above, and discuss any differences inf the ease of breaking the ethyne C-H bond by these two different processes.
q
In B1, the bond-breaking is heterolytic, whereas in
B2 the bond breaking is hemolytic.
q The ethyne C-H bond is easier to break heterolytically but harder to break homolytically. This is because in heterolytic dissociation, anion stability effects are dominant over bond dissociation energy effects.
C. Synthesis of Alkynes
1. [3 pts] Sketch an efficient synthesis of the the alkyne shown below, starting with 1-pentene. [Caution: Be specific and careful in your choice of the nature of the base used and the amount of it]
![]()
q
Graders: Do not count off for omitting water from
the b. part of the second step.
q
Note that you must start with 1-pentene.
q
You must use sodium amide (at least for one of the
elimination steps).
q
You must use 3 moles of NaNH2.
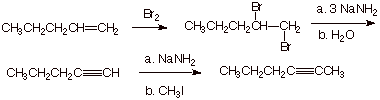
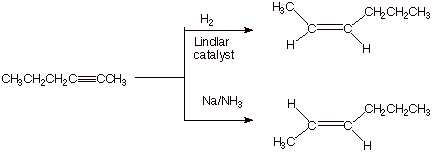
2.
[4 pts] Show by providing the
proper reagents on the scheme below how the latter alkyne (the one made in the
immediately preceding question) could be converted to cis-2-hexene and also, independently, trans-2-hexene with high selectivity ( in each case, a
single reaction).