CH 610A/618A
Final Exam
Fall, 2002
Prof. N. L. Bauld
Answer Key
__________________________________________ Name (Last,
First)
__________________________________________ Social Security Number
Question |
Possible Points |
Page/Pts/Points Earned |
Score |
|
I. |
23 |
2/12/ 3/11/ |
|
|
II. |
21 |
4/8/ 5/9/ 6/4/ |
|
|
III. |
19 |
7/11/ 8/8/ |
|
|
IV. |
25 |
9/14/ 10/11/ |
|
|
V. |
29 |
11/12/ 12/13/ 13/4/ |
|
|
VI. |
16 |
14/7 15/9/ |
|
|
VII. |
17 |
16/17 |
|
|
Totals |
150 |
|
|
I.Mechanisms of Nucleophilic Substution Reactions
A. Nucleophilic Substitution Mechanisms
1. [3 pts] Write the detailed mechanism for the reaction shown below and give the
symbolic classification of the reaction. What does the numeral signify?
![]()
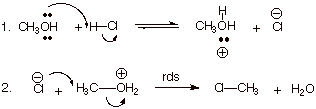
q The symbol is SN2.
q The 2 means the rds is bimolecular (involves 2 molecules).
2.
[5 pts] Provide a resonance theoretical treatment of the transition state for
the rate determining step of the reaction, summarize as a dotted line/partial
charge structure and characterize the TS model. To what kind of structural
effect is this kind of TS especially sensitive?
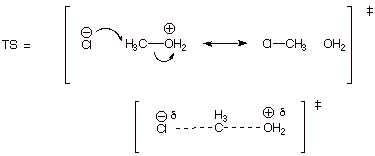
q
This TS has
pentacovalent (or pentacoordinate) carbon character
q
It is especially
sensitive to steric effects;
3.
[2 pts] Explain why the first step of the reaction is reversible and, in addition,
why it is such an essential
part of the mechanism.
q
It is reversible because
proton transfers are typically rapid (the reverse step is also a proton
transfer)
q
Protonation is essential
because this gives a good leaving group, the neutral molecule water. Without
the protonation the leaving group would have to be hydroxide anion, which is a
strong base and a poor leaving group.
4.
[2 pts] In your answer to A.1. ,
above, you presumably specified a particular substitution mechanism. Explain
why this particular alcohol, methanol, is (1)particular well suited for the
observed mechanistic type and (2) why it is particularly unsuited for the alternative mechanistic type.
q
The methyl system is the
least sterically hindered system, since hydrogen is the smallest atom.
q
The methyl system is
highly unfavorable for an SN1 mechanism because this would require
the formation of a methyl carbocation, which is a very high energy species.
5.
[3 pts] What role does chloride ion play in the mechanism? Classify the
chloride anion as strong or weak with respect to (1) basicity and (2)
nucleophilicity. What is the difference between basicity and nucleophilicity?
q
The chloride anion acts
as a nucleophile.
q
It is a very weak base.
q
It is a reasonably strong nucleophile.
B. More Nucleophilic
Substitution Mechanisms
1. [3 pts] Write the detailed mechanism for the
reaction shown below:
![]()
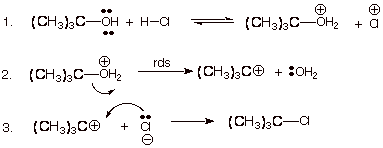
2. [3 pts] Provide a resonance theoretical treatment of the TS of the rds, summarize as a DL/PC and characterize the TS model.
q The TS has carbocation character.
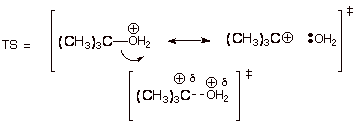
3. [2 pts] State the Hammond Postulate, and apply it to
this reaction step in order to refine your characterization.
q
The TS more closely
resembles the higher energy partner of the R and P.
q
The rds is endothermic
because it breaks a bond and forms none. Thus the TS resembles the higher
energy partner, the product of that step, which is a carbocation. The TS has extensive (or highly developed)
carbocation character.
q
II. Kinetics, Stereochemistry, and Solvent Effects
A. Kinetics
1. [2
pts] Assume that we have established that the mechanism of the nucleophilic
substitution reaction shown below is of the SN1 type. Write the rate
law for the reaction in the standard form. What is the meaning of the term
“rate constant”?
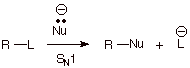
![]()
q
The “rate
constant” is the rate of a reaction at unit concentrations of all
reagents.
2. [2 pts] Now assume the mechanism for
the same overall reaction is of the SN2 type. Write the rate law for
the reaction. For each of the mechanisms involved in the preceding question and
in this question, what effect would doubling the nucleophile concentration have
on the reaction rate? Explain.
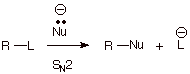
q
R = k[[R-L][Nu-]
q
Doubling the
nucleophile concentration has no effect on the rate of the SN1
mechanism, because the nucleophile is not involved in the rate law (or because
it is not involved in or prior to the rds).
q
Doubling the
concentration of the nucleophile in the case of an SN2 mechanism
doubles the rate, because the nucleophile is involved in the rds and in the
rate equation.
3. [2 pts] Now, suppose that the reaction is definitely of
the SN1 type, as in A.1,
and that the nucleophile is exchanged for one which is a much stronger
nucleophile. What effect, qualitatively, would the change to a stronger
nucleophile have on the reaction rate, assuming that the mechanism remains
unchanged? Explain.
q Still no effect upon the rate because the
nucleophile is not involved in the rds.
B. Solvent Effects
1.
[2 pts] Consider the same reaction shown above in part A.1. Provide (you need
not derive it) a dotted line/partial charge model for the TS of the rds, and
indicate what effect an increase in solvent polarity would presumably have upon
the rate. Explain your reasoning.
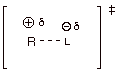
q
The TS has developed
extensive ionic (or dipolar) character, which is strongly stabilized by more
ionic solvents.
q
The rate is sharply
increased by an increase in solvent polarity.
2. [2 pt] Now provide (without necessarily deriving) a TS
model for the TS of the rds of the reaction of A.2, above, indicate what effect
an increase in solvent polarity would be expected to have upon the reaction
rate, and explain why.
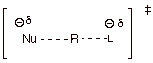
q
The reactant Nu is
negatively charge and the TS is still negatively charged. Ionic character not
developed, but rather the size of the negative ion is increased.
q
Larger ions are less
efficiently solvated.
q
The rate is decreased
to a modest extent.
3.
[2 pts] Finally, suppose that the solvent in the SN2 reaction (A.2),
is changed to dimethyl formamide or dimethylsulfoxide. What kind of effect does
this have upon the rate. Explain, and name the type of solvent represented by
these two examples.
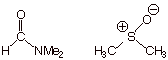
q
These are dipolar
aprotic solvents.
q
They sharply
accelerate the rate of the SN2 reaction because the
are able to solvate the positive counter ion, but leave the negatively charged
nucleophile relatively unsolvated and very reactive. (If they were protic, they
would use their protons to solvate and stabilize and render less reactive the
negatively charged nucleophile).
C. Stereochemistry.
1.
[ 3 pts] Sketch the mechanism of the
reaction shown below so as to illustrate the stereochemical outcome of the
reaction. Draw the structure(s) of the product(s) and provide the term which
describes this stereochemical outcome. Again, the “squiggly line
indicates an unspecified stereochemistry, which is for you to determine.
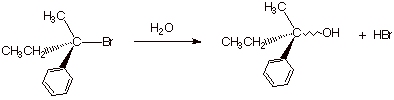
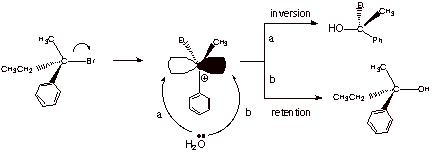
q
The stereochemical
outcome is racemization.
q
Both products are
formed because this is a tertiary system which undergoes only an SN1
reaction, via a carbocation, which is sp2 hybridized and can react
from either face with equal facility.
D. Leaving Group Ability
1.
[ 2 pts] Arrange the following leaving groups in terms of their facility as
leaving groups (1 = best), and explain the theoretical basis for your
predictions.
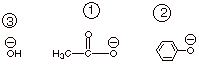
q
The best of these
leaving groups is acetate anion (the second structure) and the worst is
hydroxide anion.
q
The principle is that
the best leaving groups are the least basic groups, and hydroxide is by far
more basic than phenoxide than acetate ion.
E. Nucleophilicity.
1.
[ 2 pts] Arrange the following nucleophiles in order of their reactivity (1 =
most reactive), and explain the theoretical basis for your predictions.

q
The strongest base is
also the strongest nucleophile, provided that the nucleophilic reactivity is on
the same atom (oxygen in all three cases here).
2.
[ 2 pts] Arrange the following set of nucleophiles in order of their relative
reactivities (again, 1 = most reactive), and explain the basis for your
predictions.
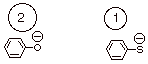
q
Although the
phenoxide anion is more basic than the thiophenoxide anion, the latter is much
more nucleophilic because reactivity is on sulfur, a much more polarizable atom
than oxygen.
III.Elimination Reactions; Competition with SN Reactions
A. Mechanism of Elimination
Reactions
1. [3 pts] Use resonance theory to derive a TS model
for the generalized elimination reaction shown below, summarize as a DL/PC
structure, and characterize the resulting TS model.
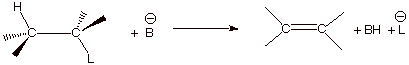
q The TS has alkene character.
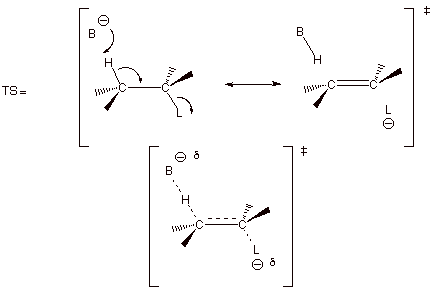
2.
[ 1 pt] Assuming that this is a
concerted reaction, what is the symbolic mechanistic designation for the
reaction mechanism?
q E2
3.
[ 2 pts] Is this reaction base catalyzed? Why or why not? What is the
appropriate term to describe the role of the base in this reaction.
q No, because the base is consumed in the reaction.
q The reaction is termed “base promoted”.
B. Selectivity in Systems in
Which There are Two Non-Equivalent Beta Hydrogens
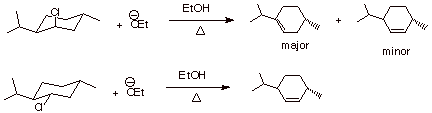
1. [2 pts] Provide the structures and names of the products of the following reaction. Designate the alpha and the two distinct beta positions of the reactant and indicate which product arises from the removal of which beta hydrogen, and which product is the major product.
![]()
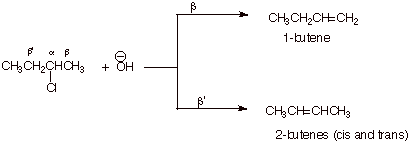
2.
[3 pts] Use
the Method of Competing Transition States to compare the TS’s for the
reactions leading to these products. Apply the respective characters of these
transitions states to explain the predominance of the product which is observed
to be major.
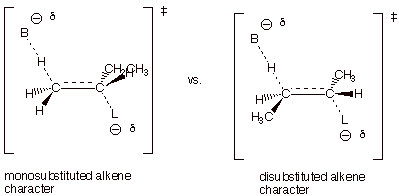
q
The TS on the left,
which leads to 1-butene, has monosubstituted alkene character, while that on
the right, which leads to the 2-butenes, has disubstituted alkene character.
q
The 2-butenes are the
major products since the more highly substituted alkene character is favored
(or alkyl groups stab ilize alkenes and alkene character).
Stereochemistry of Eliminations
1. [4 pts] Draw the structures of the products of the following
eliminations and explain, with the assistance of conformational depictions, any
selectivities which may be involved. What is the preferred stereochemistry in
these reactions ( the relationship of the beta hydrogen and the leaving group)?
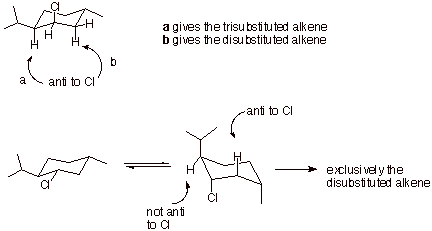
q
The first reaction
gives both products because the beta H and leaving group Cl prefer to be anti in E2 eliminations. The Cl is axial in this
reactant and is anti to an axial hydrogen on both of the neighboring,
non-equivalent carbons.
q
However, the
trisubstituted alkene is favored over the disubstituted alkene, because
trisubstituted alkene character in the TS is more favorable than disubstituted
alkene character.
q
In the second
reactant, the chlorine is equatorial in the most favored conformation, so it is
not anti to any hydrogen, but when ring flipping occurs it is axial and is anti
to just one hydrogen, that on the methylene carbon.
D. Competition Between Elimination and Nucleophilic Substitution.
1. [ 1 pt each = 2 pts] For each of the cases below,
indicate whether substitution or elimination is expected to be preferred and
explain the basis for your prediction. Provide the structure of the product(s)
of the preferred reaction.
a.
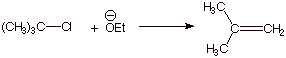
q Elimination is preferred because a strong base is used, which prefers
elimination (alternately, the tertiary system is slow to undergo SN2
substitution, but undergoes elimination readily).
b.
![]()
q Substitution is preferred because there is no beta
hydrogen to undergo elimination.
q Methyl systems are especially facile in undergoing
substitution.
2.
[ 2 pts] In the following reaction, you want to maximize the amount of
elimination and minimize the amount of substitution. Which base do you choose,
ethoxide or tert-butoxide?
Explain.
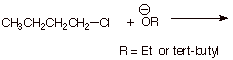
q
Tert-butoxide is the
base of choice because it is sterically hindered (bulky) and this steric effect
is important in the SN2 TS , rendering it less favorable.
q
On the other hand,
elimination is not seriously hindered, because the base is reacting at a very
small atom, hydrogen.
IV.Alcohols
A.
IUPAC Nomenclature
1. [2 pts each = 4 pts] Provide the correct and complete IUPAC names for the following alcohols.
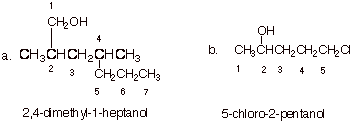
q
In part a, the OH function must be included in the
main chain, and numbering begins at the end nearest the OH groups. Be sure to
include the locant for the OH group.
q In part b, again the OH group is determining, because we are naming the compound as an alcohol (the chlorine is regarded as a substitutent).
B. Acidity of Alcohols
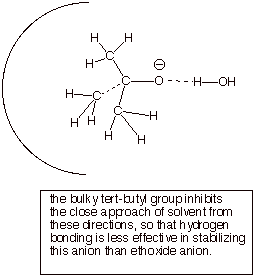
1. [3 pts] Specify the pKa’s of tert-butyl alcohol and ethanol. Is tert-butyl alcohol more or less acidic than ethanol?
Explain any difference in acidity, providing the name of the effect which
causes the difference, and a
structural illustration of the effect.
![]()
tert-butyl alcohol
q The pKa of ethanol is 16. The pKa
of tert-butyl alcohol is ca. 19.
q Ethanol is more acidic than the tertiary alcohol
because of anion stability effects.
q Steric hindrance to solvation of the hindered tert-butoxide anion makes this anion
less stable, and the corresponding alcohol less acidic.
C. Hydroxide Anion As a
Leaving Group.
1. [ 2 pts] Indicate whether the reaction written below is fast or slow
and explain the basis for your answer.
![]()
q The reaction is very slow—essentially does
not occur at all, because the OH anion is a poor leaving group.
D. Conversion of Alcohols to
Ethers
1. [ 3 pts] Write the detailed
mechanism for the reaction shown below.
![]()
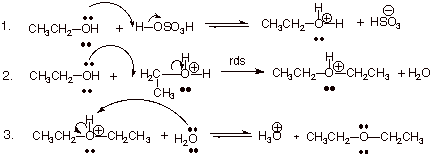
q In the first step, either sulfuric acid or hydronium ion can be used. In the third step, either water or the bisulfate anion can be used as the base, with full credit.
2. [ 2 pts] Classify this reaction as a specific type of SN reaction and explain why it is relatively slow (requires heating to above 140 o C).
q This is an SN2 reaction, because it
involves a primary carbon, which is not able to undergo an SN1
mechanism.
q It is slow because the nucleophile, ethanol, is a relatively weak nucleophile.
3. [ 2 pts] As a method for ether synthesis, is this a
good, general method for the preparation of ethers? Why or why not?
q No, because it can only be used to make symmetrical
ethers and especially symmetrical primary ethers.
E. Oxidation of Alcohols to
Carbonyl Compounds
1. [ 3 pts] Write a partial mechanism
for the oxidation reaction shown below. You need not show the mechanism for the
formation of the chromate ester, but illustrate its structure and the mechanism
for its conversion to the final product. What is the reaction type for the last step of this reaction?
![]()
q The last step is an E2 elimination reaction.
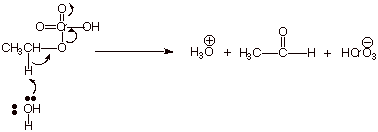
2. [ 3 pts] This same reagent does not work at all for the conversion of another primary alcohol, 1-octanol to the corresponding aldehyde (octanal). Explain why not, providing the structure of the actual product obtained and sketching the mechanism for the formation of this product (You may use R to represent the long alkyl chain (heptyl).
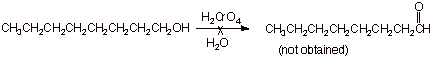
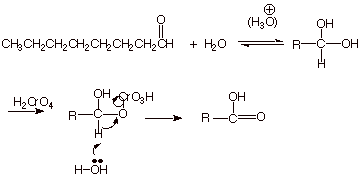
q
The initially produced aldehyde is subject to
further oxidation to the corresponding carboxylic acid. Since this is an
aldehyde with a b.p. much higher than water, it cannot be distilled off as it
is formed, as in the case of acetaldehyde, and remains in solution, subject to
that oxidation.
3. [ 3 pts] The reagent PCC (pyridinium chlorochromate) is especially effective for the conversion of alcohols like 1-octanol to their corresponding aldehydes. Explain why this reagent works when the aqueous chromic acid oxidant fails. (You need not write a mechanism).
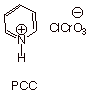
q
This reagent is soluble in dichloromethane,so that no
water is used, and therefore no
conversion to a hydrate is possible. The hydroxyl group of the aldehyde hydrate
is necessary for formation of the
chromate ester, which is essential for the oxidation. Therefore the aldehyde is
stable with respect to further oxidation.
V.Ethers
A. IUPAC Nomenclature
1. [2 pts each = 4 pts] Provide correct and complete IUPAC names for the following compounds:
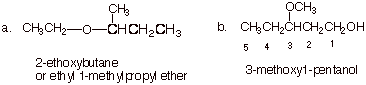
q
In the first case, standard ether nomenclature would
name both alkyl groups and sequence them in alphabetical order. The alkyl
groups are ethyl and 2-methylpropyl (sec-butyl would not be fully acceptable to
IUPAC).
q
An alternate acceptable approach would be to use the
alkoxy substituent approach, with an ethoxy substituent at the 2-position of
butane.
q
The second structure is an alcohol and requires the
use of ether substituent nomenclature (the methoxy group is the substituent).
B. Cleavage of Ethers by Hydrogen Chloride.
1. [ 4 pts] Write the detailed mechanism for the cleavage of diethyl ether by one mole of HCl.
![]()
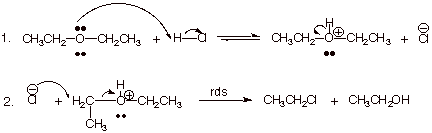
2. [ 2 pts] The overall reaction involves a step that is of the SN type. Indicate what specific sub-type of the SN reaction is involved, and explain why this sub-type is preferred.
q
This is an SN2 mechanism, because it
involves a primary carbon. (Also, chloride ion is a pretty good nucleophile).
3. [ 2 pts] Do you expect the corresponding reaction with H-Br to be faster or slower? Explain.
q Faster, because bromide anion is a better nucleophile (more polarizable) than chloride ion. (Also, HBr is a stronger acid than HCl). Will accept either answer, but give 1pt extra credit for both answers).
C. Williamson Ether Synthesis
1. [ 2 pts]Sketch two possible Williamson-type syntheses of the following ether, starting with any necessary alcohol and any necessary alkyl halide.
![]()
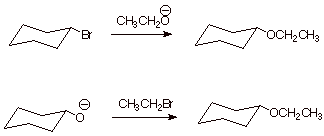
2. [ 2 pts] Indicate which of these is likely to be more efficient and explain why in mechanistic terms.
q The second one is more likely to be efficient because it involves an SN2 displacement on a primary carbon, whereas the first involves displacement on a secondary carbon, which is much slower and subject to competition with an E2 elimination.
D. “Autoxidation” of Ethers
1. [ 3 pts] Write the detailed mechanism for the air oxidation of diethyl ether to yield the indicated hydroperoxide product. You may use a general alkoxy radical (RO.) to show the initiation step.
![]()
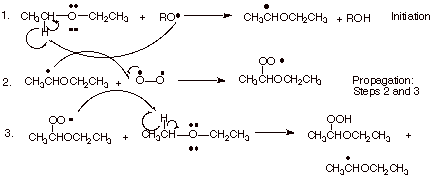
2. [ 3 pts] Indicate what type of unusual stabilization the following radical has and illustrate it by means of an energy level diagram.
![]()
q This has 3 electron bonding.
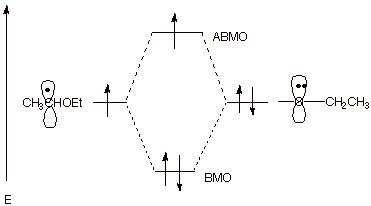
3. [ 1 pts] What does this have to do with the ease of air oxidation of ethers as compared, for example, to the same type of oxidation of alkanes?
q
This means that C-H bonds alpha to ether functions
are easier to homolyze. Ethers are therefore much easier to oxidize than
alkanes.
4. [ 2 pts] Of the two propagation steps in this reaction, which one is inherently the fastest? Explain why. What is unique about the dioxygen molecule?
q Step 2, the reaction of the radical with dioxygen is the fastest, because it is essentially a radical coupling, since dioxygen is a diradical.
E. Miscellaneous
1. [ 2 pts]Provide the name of the ether shown below, indicate which metal ion it is ably to coordinate especially powerfully, and explain why other metal ions are not coordinated nearly so powerfully.
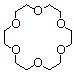
q
18-Crown-6 Ether.
q
Bonds especially powerfully to potassium because
this ion is just the right size to fit into the cavity. Other metal ions are
either too large or too small.
2. [ 2 pts] Indicate what advantage this ether has as a coordinator of metal ions in comparison with a simple ether like diethyl ether. Consider the possibility of using not just one molecule of diethyl ether, but multiple molecules.
q Crown
ethers have multiple ether functionalities in the same molecule. This is far
better than using multiple ether molecules because of the loss of translational
entropy which occurs when many individual molecules are required to be combined
with a single metal ion.
VI. Epoxides
A. Synthesis of Epoxides
1. [ 2 pts] Sketch a simple synthesis of an epoxide starting with the halohydrin indicated below, and draw the structure of the epoxide.
![]()
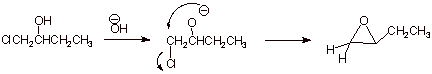
2. [ 1 pt] This reaction can be viewed as an intramolecular version of a familiar type of ether synthesis. What is the name of this type of ether synthesis.
q
Williamson Synthesis.
B. Cleavage of Epoxides in Alkaline Media
1. [ 2 pts]Simple ethers are quite stable to base, whereas epoxides are extremely reactive in basic solution. Explain why a simple SN2 reaction of a typical ether with hydroxide anion doesn’t readily occur. Then explain why such a reaction occurs readily with an epoxide.
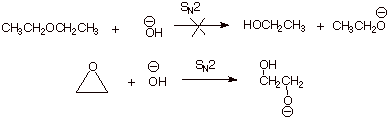
q The displacement on a simple ether doesn’t work because the ethoxide or other alkoxide anion is a poor leaving group.
q
The reaction works with epoxides in spite of this
because of the relief of a large amount of ring strain present in the
three-membered ring.
2. [ 2 pts]Provide the structure of the product(s) of the following reaction, including the stereochemistry. Explain the basis for the reaction stereochemistry in terms of a familiar stereochemical result.
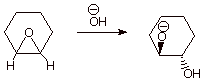
q
You may show the product as either the diol or the
conjugate base, as above. The essential item is the stereochemistry, which is
trans.
q This is essentially an SN2 reaction, so the leaving group alkoxide should be on the opposite face from the entering nucleophile, the hydroxide ion. Therefore the OH group, which comes from the nucleophile, will be trans to the alkoxide group.
3. [ 3 pts]Predict the structure of the main product of the following reaction, and explain any selectivity which is observed. What type of selectivity is this?
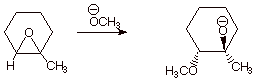
q
Again, the product can be shown as the conjugate
base (alkoxide) or as the alcohol. As before, the methoxy and alkoxy groups
must be trans and for the same reason as before. Incidentally, this places the
methyl group cis to the methoxy group.
q Additionally, we observe regioselectivity in this reaction. The methoxy group reacts preferentially at the secondary carbon of the alkoxide moiety, rather than the tertiary carbon, which is more hindered. This is as expected for an SN2 mechanism.
C. Cleavage of Epoxides in Acidic Media
1. [ 3 pts] Write the detailed mechanism for the cleavage of the epoxide of isobutene in acidic methanol.
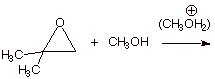
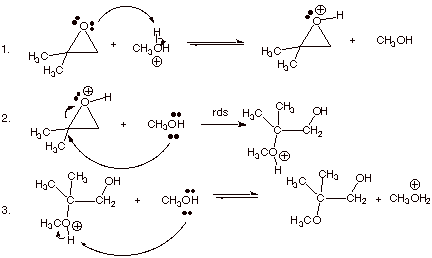
2. [ 3 pts] Provide a resonance theoretical treatment of the conjugate acid of this epoxide, summarize as a DL/PC structure and explain the preferred position at which methanol attacks this conjugate acid.
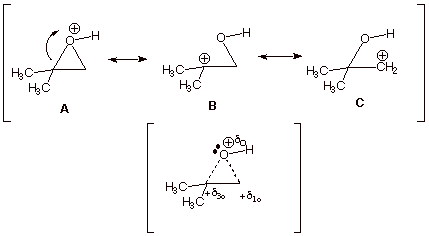
q Structures B and C show carbocation character on both epoxide carbon atoms, but structure B is of lower energy than structure C, because in B the carbocation center is tertiary and in C it is primary. Therefore B contributes more than C to the resonance hybrid, and there is more carbocation character at the tertiary carbon.
q The nucleophile tends to react preferentially at the site having greater carbanion character.
VII. Alkynes/ Synthesis
A. Nomenclature of Alkynes
1. [ 2 pts each = 4 pts] Provide correct and complete IUPAC names for the following alkynes.
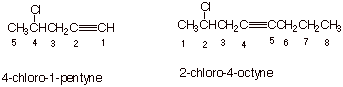
q
In the first case, it is a simple matter of
numbering from the terminus nearest the triple bond, placing the chlorine
substituent at the 4 position and the triple bond at the 1 position.
q
In the second case, the triple bond is equidistant
from both termini, so we have a tie. The tie is broken by the chlorine
substituent, which is closer to the left terminus.
B. Acidity of Alkynes
1. [ 3 pts] Cite the approximate pKa of ethyne (acetylene), and explain its high acidity in comparison to alkenes and alkanes. Your answer should include: (1) the general factor accounting for its enhanced acidity (2) the specific factor involved (3) an orbital depiction of the conjugate base of ethyne and (4) an explanation of how this factor distinguishes between the acidities of ethene, ethane, and ethyne.
![]()
q
The pKa of ethyne is ca. 21.
q
The general factor which accounts for the unusually
high acidity of ethyne is anion stability.
q
The specific factor which accounts for anion
stability is hybridization. In the ethyne conjugate base, the unshared pair is
in an sp AO, which is the lowest energy of the hybrid AO’s. In the case
of the conjugate base of ethene, the unshared pair is in an sp2 AO
and for ethane an sp3 AO. The order of stability parallels the s
content, going from 50% for the alkyne conjugate base, to 25% for the ethane
conjugate base.
![]()
2. [ 2 pts]Draw the structure of a base which is capable of generating this anion (the conjugate base of ethyne) quantitatively, and explain in quantitative terms why this base is able to remove the proton quantitatively.
![]()
q
The amide ion is the conjugate base of ammonia,
which has a pKa 38, so ammonia
is a much weaker acid than is ethyne. An equilibrium in an acid/base
reaction goes to the side of the weaker acid. This means than the amide ion
will completely convert ethyne to its conjugate base plus ammonia.
[Alternately, you could just use the equation which gives the K for the
equilibrium in terms of the Ka for the reactant acid and that for
the product acid].
3. [ 1 pts]Draw the structure of an alkyne which does not react with this same base. Explain why no reaction occurs in this case.
q
Any non-terminal alkyne, such as 2-butyne, because
it does not have a proton attached to an sp hybridized carbon. Therefore the
unshared pair in the conjugate base will not be in an sp AO.
C. Synthesis of Alkynes
1. [ 3 pts] Sketch an efficient synthesis of 1-pentyne from 1-pentene. This synthesis should involve a double elimination with an appropriate choice of base.
![]()
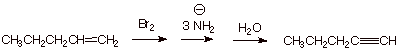
2. [ 2 pts]For which elimination step would ethoxide be sufficient? Which requires a stronger base? Why?
q
Ethoxide is okay for the first elimination, which
gives an alkene. The second elimination step, which installs the triple bond is
more difficult and usually requires a stronger base such as amide ion.
q
This is because the second pi bond of the triple
bond is not as strong as the pi bond of an alkene.
3. [ 2 pts]How many moles of base are required in order to provide a good yield of the alkyne product? Explain why this particular number is required.
q
The efficient conversion requires three moles of base, the first two to do the two elimination steps
and the third to neutralize the 1-alkyne, because it is so acidic. This
neutralization, though not desired, cannot be avoided, and consumes the third
mole of base. [Otherwise some of the two moles of base would be used to do
this]