CH 318N: Professor Nathan L. Bauld
First Exam: KEY
Spring 2005
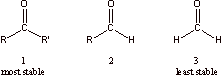
I. Carbonyl Compounds: Nomenclature, Thermodynamic Stability, Hydration
A. 1. 2-hydroxypropanal
2. 3-hexanone
3. trans-4-hexenal
B. 1.
![]()
is more stable by approximately 20 kcal/mol
2.

The carbonyl group is resonance stabilized as shown above.
C1.
C2. The carbonyl carbon has extensive carbocation character, and the additional alkyl substituents stabilize this carbocation character.
D1.
D2.
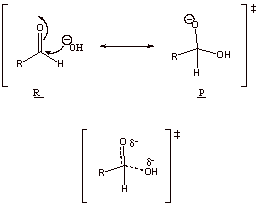
Oxyanion character
Electrostatic character
D3. The formed carbanion character on alkene pi bond is less favorable than the oxyanion character of C=O. There is no electrostatic attraction to assist H2O addition to a non-polar alkene.
![]()
Carbanion character
E1. Addition to the alkene double bond is more favorable thermodynamically as the C=C bond is weaker than C=O. Thus with alkene addition the equilibrium lies to the right.
E2. The position of the equilibrium is not dependent on whether an acid or base catalyst is used. If a reaction is under thermodynamic control, changing the catalyst will not matter as Keq is dependent only on the concentration of the reactants and products.
II. Carbonyl Compounds: Other Reactions
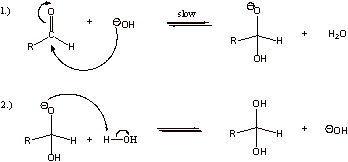
A1. Hemiacetal
A2. The reaction doesn’t proceed because hydroxyl is a poor leaving group.
A3. The reverse of the reaction does not proceed, as alkoxide is also a poor leaving group. Good leaving groups are weak bases.
A4. The second reaction has a more favorable equilibrium constant. In the first reaction, three molecules are constrained. In the second reaction, only two molecules are constrained. Converting three molecules to two in the first reaction results in a significant loss of translational entropy. In the second reaction, two molecules are converted to two molecules which results in little to no loss of translational entropy.
B1. The alternate canonical structure B is valid. Phosphorous has vacant 3d orbitals that can overlap with the filled carbon 2p orbital. This can result in a weak two electron pi bond. Structure B is higher energy structure since the use of high energy 3d orbitals is required.
B2. Phosphorous is very nucleophilic and not basic at all (polarization effect). E2 reaction requires a strong base.
B3. Formation of stable triphenylphosponoxide is the driving force. The strong ionic Ph3P+−O- bond has alkoxide character compared to the weaker Ph3P+− -CH2 bond that has carbanion character. Oxygen is more electronegative than carbon.
III. Carboxylic Acids and Derivatives
A. 3,4-Dimethylhexanoic acid
Benzoic acid
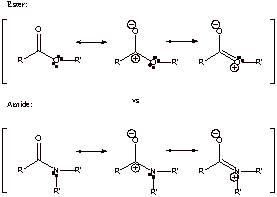
B. Ketone has only two resonance forms and no additional conjugative stabilization
Carboxylic acid has three resonance forms and it’s therefore more stable thermodynamically. Electron donating –OH is directly connected to electron acceptor C=O
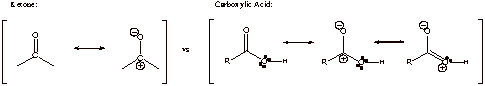
C1. The first reaction works. The second reaction doesn’t work because NaBH4 will deprotonate the carboxylic acid proton followed by formation of a very stable carboxylate anion that doesn’t react further.
C2. The carbonyl oxygen is more basic in carboxylic acid than in ketone due to resonance stabilization. Lewis acidic BH3 will coordinate to the most basic site.
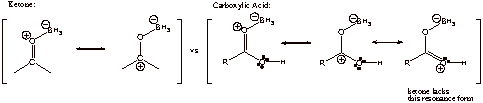
D1. Acid Catalyzed Esterification
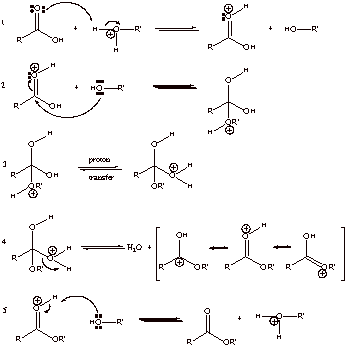
D2. Thermodynamic stabilities of RCOOH and RCOOR’ are very similar and the equilibrium constant is about 1. Excess of R’OH can drive the equilibrium to the right by removing H2O.
D3. Base-catalyzed esterification doesn’t exist. Base will deprotonate RCOOH followed by formation of a very stable RCOO- (strongly resonance stabilized). This is faster/more preferred than addition to the carbonyl group.
IV. Functional Derivatives of Carboxylic Acids
A1.
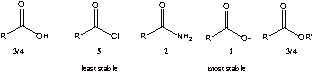
A2. N is less electronegative than O and the lone pair is more readily available to stabilize the resonance structure. The last resonance form of amide is better than the last of the ester.
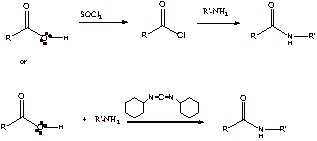
B1. Acid catalysis: Acid protonates the amine: R’-NH3+ – loss of nucleophilicity
Base catalysis: Base deprotonates the carboxylic acid – formation of very stable RCO2-
B2.
C1.
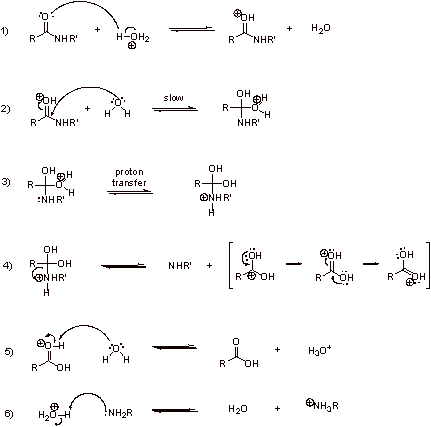
C2. Nitrogen is generally more basic than oxygen. The electron pair on nitrogen is not held as strongly to the nucleus as the electron pairs on neutral, divalent oxygen.
C3.
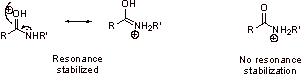
C4. In step 6, protonation of the basic amine generated in step 4 provides the thermodynamic driving force.
C5. No, this reaction is acid-promoted. In step 6, the amine is protonated and the acid is consumed.
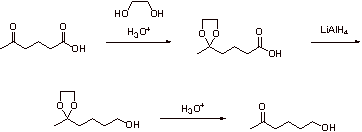
V. Synthesis and Selectivity
A.
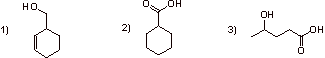
B1.
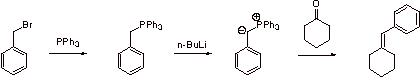
B2.
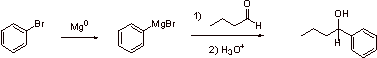
C.