Fischer Projection Structures
I. A Single Stereocenter
q
Fischer structures can
be used to simply represent absolute configurations of chiral compounds at
their stereocenters. An advantage of such structures is that they can easily
represent multiple stereocenters, and allow easy identification of planes of
symmetry, etc.
q
The Fischer structures
of (R)-2-butanol and its enantiomer are shown below.
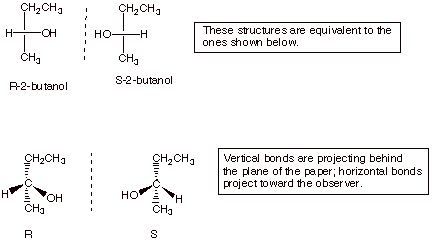
q
You are not expected to
be able to see from a given Fischer structure whether the configuration is R or
S, but if given the structure of R you should be able to draw the Fischer
structure of its enantiomer.
II. Two Equivalent
Stereocenters.
q
Given a Fischer
structure of a molecule having two stereocenters, you should be able to draw
the Fischer structure of its enantiomer and the other stereoisomers.
q
Given the configuration
of the the original stereoisomer, you should be able to designate the
stereochemistry of all of the other stereoisomers.
q
Note that the Fischer
structure implies an eclipsed conformation.
q
Consider
2,3-dibromobutane:
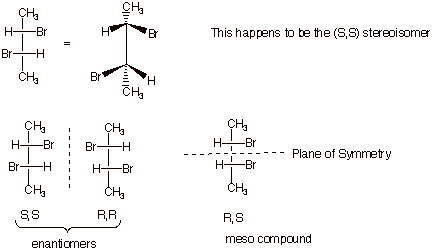
q
So, if given the Fischer
structure of say the S,S stereoisomer, you should be able to draw the structure
of its enantiomer and any other stereoisomers.
III. Two Non-equivalent
Stereocenters.
q
Consider
3-chloro-2-butanol:
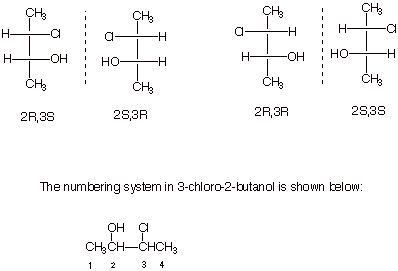
q
Again, given one
structure and its designation, you should be able to draw and designate all
other stereoisomers. Of course, reflection in the mirror reverses all
configurations. Also, interchanging any two groups inverts the configuration.