Lipids
Introduction.
Lipids are a diverse class of naturally occurring
molecules which have in common essentially only the method of their isolation,
which is by extraction of cells or tissues with a nonpolar organic solvent.
They are therefore relatively nonpolar themselves. Our discussions will center upon two main sub-groups of
lipids: (1) triglycerides and (2) terpenes and steroids.
Triglycerides
Triglycerides arenaturally occurring tri-esters of 1,2,3-propanetriol (glycerol; also called glycerin). The carboxylic acid portion of the ester is typically derived from a long chain carboxylic acid, such as stearic acid and palmitic acid.
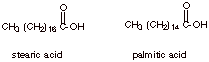
The generalized structure of triglycerides is therefore as shown below:
q In most cases, the carboxylic acids found in triglycerides have from 10-20 carbons in the chain.
q The number of carbons in the chain is virtually always an even number. We shall see why this is a little later.
q The R groups may be the same or different.
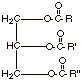
Unsaturated Acids
Although the carbon chains in many of the carboxylic
acids found in triglycerides are saturated (except for the carbon of the
carboxyl function), many unsaturated acids are found, also. In fact there may
be one, two, three, or even more C=C bonds in the chain. Two examples of the
unsaturated acids which are commonly found in triglycerides are given below:
q
Note that in most
triglycerides, the C=C’s have the thermodynamically less stable cis geometry.
q
When two or more
C=C’s are present, they are non-conjugated.
 .
.
Fats and Oils.
Naturally occurring triglycerides may be either solid
or liquid at room temperature. Commonly, solid triglycerides are called fats and liquid triglycerides are called oils. The carboxylic acid moieties involved in forming fats
and oils are often referred to as fatty acids (even numbered, C12-C18 mostly).
q
Triglycerides which have
all or most of their acidic moieties saturated are most often solid.
q
These solid
triglycerides or fats are most often found in animal triglycerides.
q
Triglycerides which have
most of their acidic moieties unsaturated are more often liquids.
q
These are referred to as
oils, and they are more commonly found in plant triglycerides.
q
There are exceptions to
these generalities, e.g., some plants have large amounts of saturated triglycerides.
q
A simple explanation of
why unsaturated triglycerides have lower m.p.’s (i.e., are liquid at room
temperature) is found in the ease of packing of the respective molecules into a
snug crystal lattice.

q
The all anti saturated alkane linkages fit together nicely and
provide close approach and favorable van der Waals attractive forces.
q
The cis double bonds hinder close approach and diminish the
van der Waals interactions (see dotted lines for repulsions which impede close
approach of the rest of the chain. They also prevent the formation of extended,
linear lattice.
q
Interestingly,
unsaturated triglycerides which have trans double bonds tend to have higher m.p.’s than the
ones with cis double bonds, and
thus to be solid at room temperature. They, like the saturated analogues, tend
to pack densely into a rather linear, extended lattice.

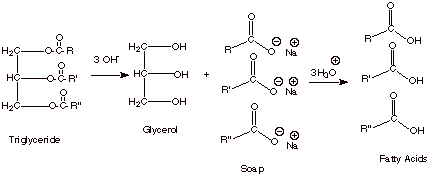
Hydrolysis of
Triglycerides
Since triglycerides are esters, they are readily
hydrolyzed via either acid catalysis or base promotion. Base promoted
hydrolysis is of special importance and will be considered exclusively here.
q
When 3 moles of
hydroxide ion are used to hydrolyse a mole of triglyceride in aqueous solution,
the products are one mole of glycerol and three moles of sodium salts of the
carboxylic acids which were present in bound form in the triglyceride.
q
Acidic aqueous workup
gives the carboxylic acids, themselves. These naturally occurring acids are
often called “fatty acids”.
q
Since fats and oils are
available in abundance, this hydrolysis can be used to produce glycerol and
either the sodium salts of carboxylic acids or the fatty acids, themselves.
q
The sodium salts are
especially useful because they constitute “soap”.
Soaps
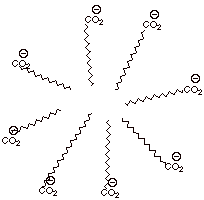
Soaps
exert their cleansing action in aqueous solution because of the formation of
fundamental particles called micelles.
q
An illustration of the
structure of a soap micelle is
shown below. Note that the interior of the micelle is nonpolar because it contains all of the nonpolar hydrocarbon
“tails” of the soap.
q
The exterior of the
micelle is highly polar because it
contains all of the ions: the negative carboxylate ions and the sodium ions.
These are nicely solvated and stabilized by the surrounding solvent molecules.
The nonpolar “tails” are protected from the water, which which they
would not interact favorably.
q
The hydrocarbon tails
interact with each other favorably by van der Waals attractions.
q
The result is that
nonpolar substances like grease are able to enter the interior of the micelle
and be favorably solvated by the nonpolar tails. In this way, substances which
would not dissolve in water at all, are able to be included inside the aqueous phase.
q
Note that micelles are
not considered to be dissolved in
the ordinary sense, because they are rather large particles, but neither are
they considered to be suspended
particles. They are intermediate species. Typically, micelles might contain
50-200 molecules in a roughly
spherical shape.
q
It turns out,
interestingly, that the length of the hydrocarbon tails which are present in
naturally occurring fatty acids, are just right for forming stable micelles, which are essential to cleansing action.
q
If the fatty acid salt
has less than 12 carbons, the van der Waals attractions between the tails is
not large enough to afford a stable interior for the micelle. If it is more
than about 18 carbons, the van der Waals interactions are too strong, and they
result in precipitation of the salt, with the tails interacting more strongly
in the solid, insoluble lattice.
q
Potassium salts can also
be used. They tend to be softer solids (or even liquids) than sodium salts.
Detergents
q
The basic principle
behind the cleansing action of soap is the ability to form micelles with a nonpolar interior.
q
This merely requires
molecules which have a highly polar part and a nonpolar part. Of
course, the nonpolar part must have a length and shape which will permit the
formation of a stable micelle.
q
For example, the ionic
part could be an anion other than a carboxylate anion. Synthetic cleansers,
known as detergents, have been synthesized which use the sulfonate anion as the
ionic part.
q
By doing this, it has
been found that sulfonate based detergents are compatible with “hard
water” (i.e., water which contains dissolved calcium and magnesium ions),
whereas soap is not.
q
In the presence of
either of these ions, soap exchanges its counterion and precipitates out of
solution as the insoluble calcium or magnesium salt of the fatty acid. However,
the sulfonate salts of calcium and magnesium are soluble, so these ions do not
interfere with micelle formation.
.
![]()
q
See if you can devise an
efficient commercial synthesis of the detergent shown above, starting with
benzene and using the typical electrophilic aromatic substitution reactions
which we studied earlier in the semester. [How do you install an alkyl group? A
sulfonic acid moiety? Which should go first, alkylation or sulfonation?]
Terpenes and Steroids
Terpenes
are naturally occurring organic compounds which are ultimately formed from acetyl
coenzme A via isopentenyl pyrophosphate, the structure of which is shown below.
q
Since isopentenyl
pyrophosphate is the ultimate source
of all the carbon atoms in terpenes, they typically have a total number of
carbon atoms which is divisible by five.
q
The simplest terpenes
(we can call them monoterpenes), therefore have 10 carbon atoms. The next higher series have 15 carbons . These are
called sesquiterpenes (these have
1.5 terpene units). The next
groups has 20 carbons and are called diterpenes. Terpenes which have 30 carbons are called triterpenes.
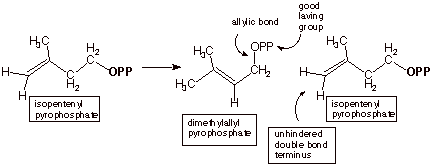
q
The mechanism for the
formation of the basic terpene building block is shown below. The starting
point is isopentenyl pyrophosphate. A molecule of this reactant is first
isomerized (the disubstituted double bond is isomerized into a more stable
trisubstituted double bond; detailed mechanism not given here).
q
At this point the
pyrophosphate moiety, which is a good leaving group, is allylic and reactive. In an SN substitution, the double bond of a
molecule of isopentenylpyrophosphate acts as a nucleophile (at its unhindered
terminal carbon) to displace the pyrophosphate moiety from the allylic
position, and generate a carbon-carbon bond which links the two molecules, forming a ten carbon pyrophosphate.
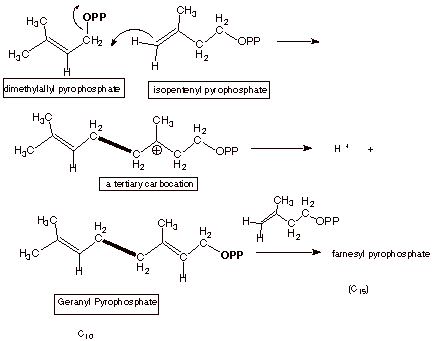
q
The 10 carbon molecule
is called geranyl pyrophosphate.
This molecule is considered to be the key intermediate for the formation of all
monoterpenes, and by further reaction with another molecule of
isopentenylpyrophosphate, as a precursor for forming sequiterpenes and
diterpenes. We will discuss the mechanism of formation of triterpenes further
along.
q
We might note that the
pyrophosphate anion (P2O6-4 ) is
generally considered to be nature’s choice of leaving group.
THE ISOMERIZATION STEP
THE CARB0N-CARBON BOND FORMING STEPS
q
As was noted above,
geranyl pyrophosphate is considered to be the precursor for all monoterpenes.
An example of the formation of limonene, an essential oil present in lemons, is
shown below.
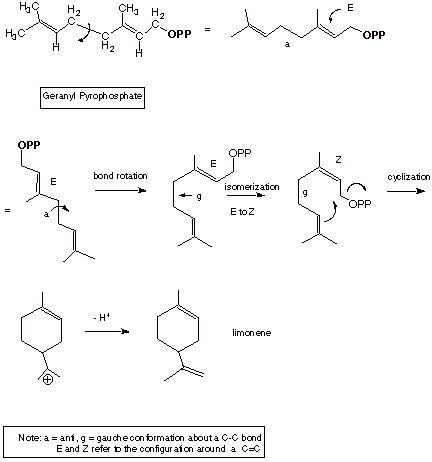
q
It was noted above,
also, that the C15 pyrophosphate, farnesyl pyrophosphate, is the direct precursor of all
sesquiterpenes.
q
Incidentally, the
triterpenes, which are closely related to the steroids, are not formed by
continuing to add isopentenyl pyrophosphate molecules one at a time. Instead,
two farnesyl pyrophosphate molecules are coupled together to give a C30
molecule which is the precursor of triterpenes and steroids. This molecule is
called squalene.
q
The structure of
squalene is given below, but the biochemical mechanism for its formation is not
covered here.
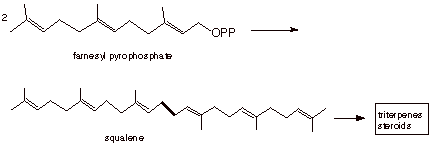
STEROIDS
The
polyunsaturated molecule squalene undergoes a unique and impressive cyclization
reaction which leads to polyclic triterpenes and steroids. We will not examine
this mechanism in detail, although it has been elegantly worked out, especially
by Professor E.J. Corey (Harvard), but it is a carbocation mechanism not unlike
we have seen for the formation of limonene, except that several rings are
formed consecutively.
q
Steroids are naturally
occurring molecules which typically have the tetracyclic polycyclic skeleton shown below. It may
be noted that cholesterol and other steroids do not have 30 carbon atoms; they
have lost a few, but they are derived biochemically from the triterpenes. Also,
steroids have additional functionalities which triterpenes lack.
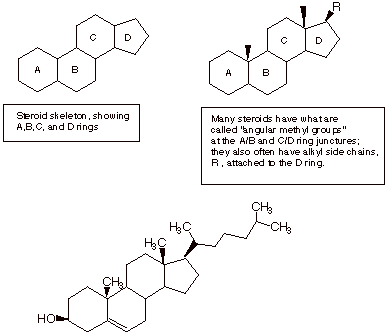
Cholesterol
A conformational structure which better reveals the specific conformation and configuration of cholesterol is also given below:
