Problem Set #2
1. Use the circle mnemonic
(the Frost-Musulin mnemonic) to arrive at a display of orbital energies for the
cycloheptatriene system. Populate the orbitals appropriately for the cation,
and calculate its resonance energy. Do the same for the anion. The MO energies
are: a+2b, a+0.62b,a+0.62b,a-0.44b,a-0.44b,a-1.80b,a-1.80b.
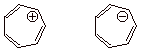
2. Using the concept of TS
aromaticity/antiaromaticity, explain why carbocation rearrangments are so
facile, and neither radicals nor carbanions rearrange at all. Supply an orbital
picture, along with your explanation.
3. Again, using TS
aromaticity/antiaromaticity, explain (with the assistance of orbital pictures),
why SN2 reactions prefer inversion over displacement. Do the same
using FO (HOMO/LUMO) theory, taking into account orbital enlargement.
4. Use FO theory, with
appropriate orbital pictures, to show why the Diels-Alder reaction of
1,3-butadiene (s-cis conformation) with ethylene is a facile reaction.
5. Employ the theory of TS
aromaticity/antiaromaticity to predict the preferred mode of approach of
carbene (CH2) to ethylene.
6. Extra Credit: Explain what
is meant by a Mobius conjugated system, and indicate such an orbital system in
the TS for carbene addition to alkenes. (We will later see another example in
the context of electrocyclic reactions).
Note: In the notes, I had
suggested that you calculate the resonance energies of the cyclopentadienyl
anion, radical, and cation, but I didn’t give you the MO energies. In
case you are still interested in doing this as an exercise, the energies are: a+2b,a+0.62b,a+0.62b,a-1.62b,a-1.62b. Also,
please be aware the HMO energies and coefficients can be obtained for virtually
any stem from “Dictionary of Pi Electron Calculations” by Coulson
and Streitweiser (A 2 Volume Set).