RADICAL CHAIN ADDITIONS TO ALKENES
We have previously discussed
electrophilic additions to alkenes (AdE) in the form of the addition
of hydrogen halides, water (acid catalyzed hydration), and bromination
(addition of Br2). We already know that additions to pi bonds are
usually thermodynamically feasible because the pi bond is weaker than most
sigma bonds which would be formed. Most recently, we have discussed radical
chain mechanisms in the form of homolytic substitution reactions (SH),
specifically chlorination and bromination of alkanes. Now we want to put
together the radical chain mechanistic concept with the concept of overall
addition to alkenes, i.e., radical chain additions to alkenes (AdH).
Our specific example is the hydrobromination of alkenes. More specifically we
will consider the addition of HBr to propene as our prototype example.

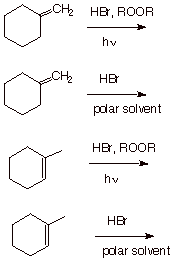
You may recall that the electrophilic addition of HBr to propene, which proceeds via carbocations, follows the Markovnikov Rule, i.e., it gives 2-bromopropene exclusively. So the radical chain addition of HBr is also regiospecific, but has the opposite sense of regiospecificity, which we call anti-Markovnikov regiospecificity. By the way, we will address the question of what “radical conditions” means, i.e., what conditions are necessary in order to bring about the radical chain addition in preference to the competitive electrophilic addition.
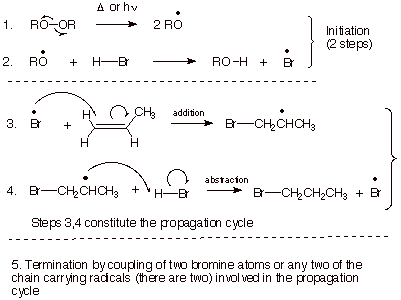
The
mechanism of the radical chain addition is shown below (although the
termination steps are omitted). You should note the following points about the
mechanism.
q
The peroxide (ROOR) is
used as an initiator. The weak O-O
bond allows this bond to be cleaved homolytically under mild thermal conditions.
q
The resulting alkyoxy
radicals are highly reactive and
abstract a hydrogen atom from a molecule of HBr, to yield bromine atoms (which
are also radicals).
q
The propagation cycle
consists of two reactions, repeated alternately, i.e., addition of bromine
atoms to the pi bond of the alkene, yielding a bromoalkyl radical, followed by
abstraction of a hydrogen atom from HBr by this radical.
q
The first step of the
propagation cycle is feasible because the newly formed C-Br bond is stronger
than the pi bond which is broken (Use your equation for calculating approximate
enthalpies of reaction in terms of the D’s of the bonds broken and
formed..
q
The second step is
feasible because the newly formed C-H bond is stronger than the H-Br bond (D
for this bond is ca. 87 kcal/mol).
q
Radical chain reactions
typically do not have a single rds, but in this case the addition of bromine
atoms to the pi bond is of special importance because it determines the
regiochemistry.
q
Note that bromine atoms
prefer to add to the less substituted end of the double bond, just as protons
did, etc. We shall see why in terms of a TS model.
Transition State Modelling
We
now use resonance theory to describe the TS for the product determining step
(the pds):
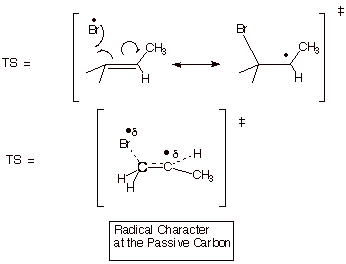
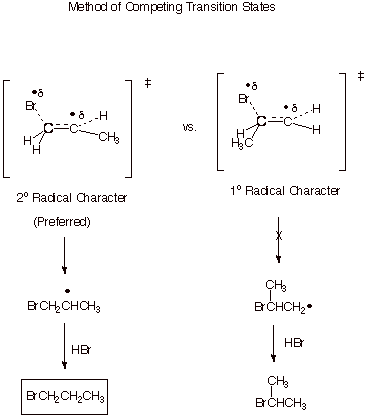
REGIOCHEMISTRY. To rationalize the preferred, anti-Markovnikov, regiochemistry of the addition we use the Method of Competing Transition States.
RADICAL CONDITIONS:
Since HBr can add to alkenes via either a radical chain or an electrophilic mechanism and since the products are different, how can we control which mechanism operates so that we can obtain the specific product which we desire? The conditions which are conducive to a radical chain mechanism are as follows:
q Presence of peroxides (either by intentionally adding them or inadvertent presence as impurities in the solvent) as necessary initiators.
q Presence of light or heat to cleave the initiator.
q Use of a nonpolar solvent (polar solvents favor the formation of ions).
To favor the ionic (electrophilic) mechanism, the following conditions are desirable:
q Purified solvents (no peroxides).
q Absence of light or heat (cover the reaction vessel with dark cloth if necessary).
q Use of an ionic solvent.
q Radical inhibitors can be added to intercept chain-carrying radicals and stop the radical chains.
SYNTHETHIC EXAMPLES.
Predict the structures of the products of the following reactions, including the stereochemistry and the regiochemistry (radical additions are non-stereospecific because the radicals are planar, like carbocations; but they are anti-Markovnikov regiospecific).