Statistical Effects Can Be Important in Unselective
Reactions Like Chlorination
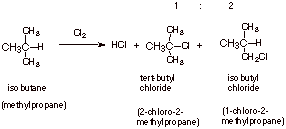
q
The chlorination of
alkanes is quite unselective, but
the inherent order of reactivity
is tertiary hydrogens greater than secondary, greater than primary (the
magnitudes of the preferences, however, are small). However, note that in the
chlorination of isobutane, the amount of the product corresponding to
substitution at the primary hydrogen actually exceeds the amount of product
corresponding to substitution at the tertiary hydrogen. The reason for this is
a statistical (probability) effect.
q
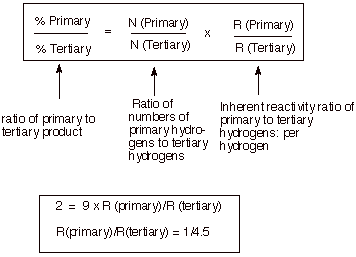
Since there are nine
times as many primary hydrogens (see three methyl groups) as tertiary, the probability that a
chlorine atom will encounter (collide with) a primary hydrogen is statistically
nine times as great as with a tertiary hydrogen. Since the inherent preference
for tertiary over primary is less than the factor of nine, there is slightly
more primary chloride produced than tertiary chloride.
q
Note that the tertiary
hydrogens are, per hydrogen, more reactive than the primary hydrogens, per
hydrogen, by a factor of 4.5.