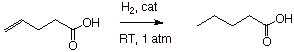CHAPTER 17. CARBOXYLIC ACIDS
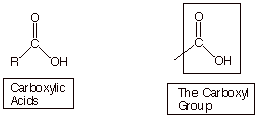
Carboxylic acids are a class of organic compounds which contain the carboxyl
functional group. They are the most
common class of acidic organic compounds.
q
In rapidly writing
structures, the carboxyl group is sometimes written as –CO2H .
q
The carboxyl
functionality is a compound functional group, containing both a carbonyl functionality and a
hydroxyl functionality. However, this compound functionality is very different
from either an alcohol or a ketone.
q
Because the carbonyl and
hydroxyl groups are directly attached to one another, there is a strong
resonance interaction between the two
groups, which sharply modifies the properties of the compound functionality
from that of either of the two component functionalities.
Nomenclature.
q
The IUPAC
approach to naming carboxylic acids (which we will primarily focus upon)
consists essentially of naming the subject compound as an alkanoic acid having
various substituents at various positions.
q
The suffix –oic is added to a stem corresponding to the longest continuous
chain of carbon atoms which contains the carboxyl group. The carboxyl carbon is
automatically numbered as the first carbon of that parent chain. The stem consists of the name of the
alkane containing the same number of carbon atoms, except that the terminal
–e of the alkane is dropped (e.g., methane becomes methan-).
q
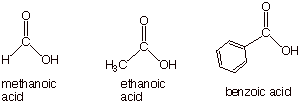
The simplest
carboxylic acid is methanoic acid, the next ethanoic acid, etc. Benzoic acid has a special, IUPAC-approved name, since
aromatic carboxylic acids are not easily named by the standard system.
q
Substituent
names and positional locants, arranged alphabetically, are added as appropriate
for the subject compound.
![]()
q
In di- or
polyfunctional molecules, the carboxyl function takes precedence over most
other functional groups for nomenclature purposes. In the example shown below,
the molecule is named as a carboxylic acid, rather than as an alcohol.
![]()
q
Note that,
since the carboxyl carbon atom is always numbered as carbon-1, a locant is not
used for the carboxyl group.
POLARITY,
SOLUBILITY, AND BOILING POINTS.
q
The carboxyl
group is considered to be a very highly polar functional group. It not only has a highly polar C=O bond,
but also a highly polar –OH bond.
q
The lower
molecular weight members of the carboxylic acid family, especially methanoic
(formic) and ethanoic (acetic) acids, are sufficiently polar as to be
completely soluble in water. As solvents, they are considered to be polar
solvents, but not quite
as polar as water. Solvent polarity is often measured by the ability to
dissolve salts or to provide solvation stabilization for ions.
q
Higher molecular weight carboxylic
acids, tend to have less water solubility because of the dominance of the
non-polar part of the molecule, which is “hydrophobic”. They are
therefore more organic-soluble.
q
The carboxyl
group has both an acidic and a basic site. The acidic site is the protonic end
of the O-H bond, and the most basic site is the carbonyl oxygen atom. As such, the
carboxyl group is both a hydrogen bond donor and a hydrogen bond acceptor. Consequently, the neat (pure) liquids
are highly hydrogen bonded intermolecularly (i.e., between molecules) and
therefore have especially high boiling points for their molecular weights.
RESONANCE STABILIZATION
q
As noted
above, the carboxyl group is a compound functionality in which a hydroxyl group
(an electron donor via the unshared pair on oxygen) and a
carbonyl group (an electron acceptor because of the carbocation character of the carbonyl carbon atom)
are directly connected. The result is strong resonance stabilization of the
carboxyl group via the interaction between these two groups.
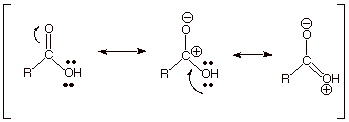
ACIDITY.
In
a previous chapter, the acidity of carboxylic acids was considered in detail. A
brief review of a few of the essential points about carboxylic acid acidity is
presented here.
q
The pKa’s
of simple carboxylic acids, such as acetic acid, are typically about 5.
q
A comparison
with the acidity of another class of organic compounds containing the hydroxyl
group, viz., alcohols is instructive. Simple alcohols, such as ethanol, have pKa’s
of about 16, fully 11 pKa units less acidic than carboxylic acids,
although in both cases it is an O-H bond which undergoes dissociation. Incidentally,
11 pKa units corresponds to a free energy difference of ca. 15
kcal/mol.
q
What is the
reason for the much greater acidity of the OH protons of a carboxylic acid than
those of an alcohol? Primarily the
resonance stabilization of the conjugate base of a carboxylic acid, i.e., the
carboxylate anion. Recall that there are two equivalent resonance structures
for this anion, so that resonance stabilization is especially strong.
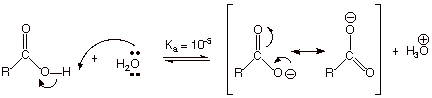
q
In contrast, the
conjugate base of an alcohol, an alkoxide anion, is not resonance stabilized at
all, i.e., the negative charge is
fully localized upon the oxygen atom.
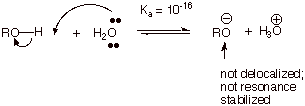
q
Preferentially
stabilizing the right hand side of the equation, results in an equilibrium
shift to the right side, and the formation of more hydronium ion.
q
However, our
argument has, to this point, neglected one important issue, viz., the
resonance stabilization of the carboxylic acid itself. Since this stabilization relates to the
reactant (left hand) side of the equation, it is a factor which should cause
the equilibrium to shift to the left, that is, it should cause a decrease in
acidity.
q
We therefore
have one factor, resonance stabilization of the conjugate base, which tends to
increase acidity, and one factor, resonance stabilization of the reactant
carboxylic acid, which tends to decrease acidity. Which factor is dominant?
q
Experimentally,
we can see that, by the large factor of 15 kcal/mol, the resonance
stabilization of the anion dominates the resonance stabilization of the
carboxylic acid. This is because the experimental result is that the acidity of
the carboxylic acid is actually increased.
q
Since the
carboxylic acid itself is strongly resonance stabilized, we can see the the
resonance stabilization of the carboxylate anion is very large indee, i.e.,
fully 15 kcal/mol greater than that of the carboxylic acid.
q
Is this
result predictable theoretically? Decidedly, yes.
Simply recall that the two best resonance structures of the carboxylate anion
are equivalent, and therefore provide a maximum resonance stabilization. In the
case of the carboxylic acid, the resonance structures are non-equivalent. In
particular, the other structures have charge separation, which is an
energy-increasing factor.
BASICITY OF CARBOXYLIC ACIDS
q
The
carboxylic acid function, besides possessing an acidic proton, has two non-equivalent basic sites. These are the carbonyl
oxygen and the alcohol type oxygen.
q
While
carboxylic acids are much more acidic than basic, these basic sites are important as
hydrogen bond acceptor sites in the intermolecular hydrogen hydrogen bonding
which gives rise to the relatively high boiling points of carboxylic acids.
q
The basic
oxygen sites, especially the carbonyl oxygen, are, however, accessible to
protonation in acidic aqueous solution containing strong acids. In fact, these
carbonyl oxygens are even more basic than those in ketones or aldehydes. This
will prove to be important when we look at the diborane reductions of
carboxylic acids further along.
q
First: Why
is the carbonyl oxygen more readily protonated than the ether type oxygen? Note that the carbonyl oxygen-protonated
conjugate acid of a carboxylic acid is highly resonance stabilized (see below), while the alcohol
oxygen-protonated conjugate acid is not.
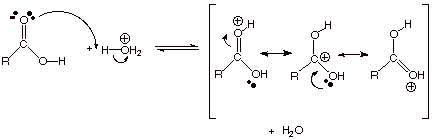
q
Further, the
latter conjugate acid has a center of positive charge (the protonated alcohol
oxygen) directly adjacent to the highly electron deficient carbonyl carbon,
which has a large partial positive charge. The electrostatic repulsion between these two directly attached sites
is therefore strongly destabilizing to the alcohol oxygen-protonated conjugate
acid.
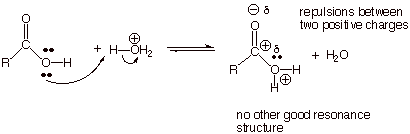
q
Second: Why
is the carbonyl oxygen of a carboxylic acid more readily protonated than the
corresponding carbonyl oxygen of an aldehyde or ketone? Again, please note that the conjugate acid
of a carbonyl compound lacks the third resonance structure which is significant
in the stabilization of the conjugate acid of the carboxylic acid. Essentially,
the conjugate acid of a ketone has partial positive charge on just two atoms,
the carbonyl oxygen and the carbonyl carbon, while the conjugate acid of the
carboxylic acid has the postive charge delocalized on three atoms. These are
the carbonyl oxygen, the carbonyl carbon, and the alcohol type oxygen.
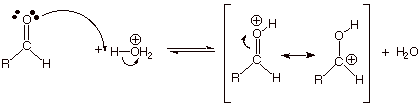
PREPARATION OF CARBOXYLIC ACIDS.
We have previously discussed two methods (at least) for preparing carboxylic acids. We want to briefly review these two methods.
The Oxidative Approach.
q
In the
chapter on the alcohol functional group, we saw that primary alcohols could be oxidized to aldehydes by aqueous chromic acid oxidation, but also that aldehydes, if
not quickly removed from solution, would rather quickly be further oxidized to
carboxylic acids.
q
In employing
the term “oxidation” in the context of organic reactions, it is
important for us to remember that carbon is considered to exist in various
possible oxidation states ranging from that present in alkanes (the zero
oxidation state), to that in alcohols or ethers (the +1 oxidation state), to
aldehydes and ketones (the +2 oxidation state), to carboxylic acids and esters
(the +3 oxidation state), and finally to carbon dioxide (the +4 oxidation
state). These oxidation states can be considered as reflecting the number of
bonds to oxygen that a given carbon has and the fact the carbon is the positive
end of the polar covalent bond, with oxygen being the negative end (in each
case oxygen has the –2 oxidation state).
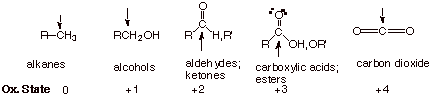
q
Any
conversion which increases the number of C-O bonds is considered an oxidation
(any conversion which generates a product which is further to the right on this
list). Any conversion which decreases the number of C-O bonds is considered a
reduction.
q
Consequently,
the conversion of a primary alcohol to an aldehyde is considered an oxidation,
as is the conversion of an aldehyde to a carboxylic acid.
q
On the other
hand, the conversion of a carboxylic acid to an aldehyde or an alcohol is
considered to be a reduction.
q
Oxidizing
agents, i.e., agents which are able to bring about oxidation reactions, are
often inorganic reagents containing atoms (especially metals) in a high
oxidation state, which can be converted to a lower oxidation state. Thus,
chromic acid, in which chromium has an oxidation state of +6, is an appropriate
oxidant for the conversion of either a primary alcohol or an aldehyde to a
carboxylic acid. In the process chromium is converted to the +3 oxidation
state.
q
The
mechanisms of these reactions were considered in the first semester of this
course and are not of further concern to us here. Please remember,however,
that, unlike aldehydes, ketones
are not easy to oxidize to carboxylic acids.
A Synthesis of
Carboxylic Acids Which Involves Reduction.
q
Only carbon
dioxide contains carbon in a higher oxidation state that carboxylic acids. The
reaction of Grignard reagents (nucleophilic reagents) with carbon dioxide
generates carboxylate anions, which upon acidic aqueous workup yield carboxylic
acids.
REACTIONS OF CARBOXYLIC ACIDS
q
By far the
most common reactions of carboxylic acids are deprotonation (that is dissociation of a proton) and protonation. Both of these have already been
discussed rather extensively.
q
Further
oxidation of a carboxylic acid (which requires very strenuous thermal oxidation
conditions) can only yield carbon dioxide. Consequently, most of the reactions
of carboxylic acids either involve reduction to an aldehyde or an alcohol, or they are
not red-ox reactions at all. For example, we have noted that carboxylic acids
and esters are at the same oxidation level. The conversion of a carboxylic acid
to an ester therefore does not involve either oxidation or reduction.
Reduction
Reactions.
Catalytic reduction.
1. We know that various unsaturated
functionalities can be reduced by dihydrogen in the presence of transition
metal catalysts.
2. Alkenes, having the weakest pi bonds, are
easiest to reduce.
3. Carbonyl groups and aromatic rings are
also reducible, but under more strenuous conditions of temperature and
pressure.
4. Carboxylic acids have a carbonyl pi bond
and, further, they have additional resonance stabilization. Consequently, they
are harder to reduce than either alkenes or aldehydes/ketones.
5. The sequence of ease of reducibility is
alkenes > aldehydes, ketones>carboxylic acids, aromatics.
6. Therefore, an alkene or an aldehyde/ketone
function can be reduced selectively in the presence of a carboxylic acid
moiety, without affecting the latter.
An Example:
Metal Hydride Reductions
q
The electrophilic
carbonyl carbon of an aldehyde or ketone makes it reactive toward metal
hydrides, which contain nucleophilic hydrogen.
q
The same is true for a
carboxylic acid, except the latter is more highly resonance stabilized than an
aldehyde or ketone and therefore inherently less reactive than a carbonyl
compound.
q
There is an even more
serious problem with the reduction of a carboxylic acid by a hydride such as
lithium aluminum hydride, i.e., the acid is acidic and prefers to very quickly
protonate the basic hydride reagent, giving dihydrogen and the carboxylate salt
of the metal. The negatively charged carboxylate anion is highly resonance
stabilized and reacts with only the most reactive hydride reagents and even so
with some difficulty.
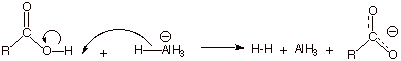
q
Nevertheless, under
these pressing conditions, carboxylic acids can be reduced to primary alcohols.
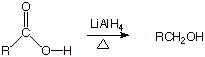
q
IMPORTANT: Keep in mind
that alkene pi bonds, which are not electrophilic, are not reduced by (nucleophilic) hydride reagents at all,
so even carboxylic acid functionalities can be reduced selectively in the
present of an alkene function.
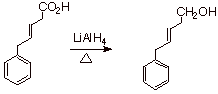
Selective Reduction of the
Carbonyl Function in the Presence of a Carbonyl Function: Borane Reductions
There is an especially clever strategy for
the selective reduction of a carboxylic acid function even in the presence of
an aldehyde or ketone function. Recall that this could not be accomplished with
LAH or other metal hydrides, because the carboxylic acid function is resonance
stabilized and therefore less reactive than a carbonyl function, and also
because initial reaction of a hydride reagent with a carboxylic acid is an
acid/base reaction which generates dihydrogen and the extremely unreactive,
highly resonance-stabilized carboxylate anion. Strategies like this are
important in organic synthesis in enabling one to direct reaction toward an
inherently more stable, generally less reactive functionality.
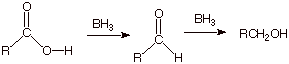
q
Note, first,
that borane is not a metal hydride. Boron is not a metal, and the hydrogens of
borane are not especially hydridic in character. Consequently, there is no
Bronsted acid/base reaction which converts the carboxylic acid to a very
unreactive carboxylate anion, as
occurred in the case of LAH.
q
Rather,
borane is electrophilic, since the boron has a vacant 2p AO. The reaction
between borane and a carboxylic acid is a Lewis acid/base reaction in which
borane is the Lewis acid and the carboxylic acid (via the unshared pairs of the carbonyl
oxygen: recall that this is the more basic oxygen) is the Lewis base.
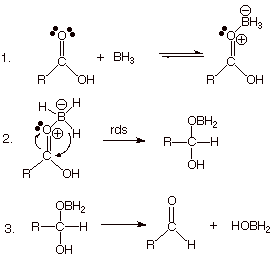
q
This
reaction converts the neutral, electrophilic borane to a negatively charged
hydride reagent, in which the hydrogens are now quite nucleophilic!
q
Simultaneously,
it converts the carboxylic acid to its (Lewis) conjugate acid (a postively
charged, oxonium ion), which (like the corresponding Bronsted conjugate acid)
has much more carbocation character at the carbonyl carbon. The activated hydride
is then able to react very
efficiently at this activated electrophilic center.
q
In the
product, which is analogous to the hydrate of a carbonyl compound, the former
carboxyl carbon has been reduced to the +2 oxidation state, which is the same
as that of a carbonyl compound. Loss of HOBH2 generates the
aldehyde.
q
If
conditions are controlled carefully, the aldehyde can be isolated in good
yield. However, if desired, this can be reduced further with an excess of the
borane to the alcohol. But remember, the carboxylic acid is more reactive
than a carbonyl compound, so this reaction can be stopped at the aldehyde stage.
q
Why is
the carboxylic acid function, which is imore highly resonance stabilized than
an aldehyde function, more
reactive than the aldehyde function? Simply because the carboxylic acid is more
basic. Recall the previous discussion of the basicity of carboxylic acids,
which revealed that the conjugate acid of a carboxylic acid has the positive
charge delocalized over three atoms, the carbonyl oxygen, the carbonyl carbon,
and the alcohol-type oxygen. So borane preferentially forms the complex with
the more basic carbonyl oxygen of the carboxylic acid.
ESTERIFICATION OF CARBOXYLIC ACIDS
Many reactions of carboxylic acids
involve neither oxidation nor reduction. That is, the oxidation state of the
carboxyl carbon remains at +3. The conversion of a carboxylic acid to a
carboxylate ester is one such reaction.
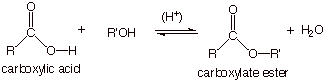
q
In an
overall reaction sense, this is a substitution reaction, since the –OH group is
replaced by an –OR’ group.
q
The
substitution reaction does not, however, occur via an SN1 or an
SN2 mechanism. Rather, it occurs in a two stage mechanism involving,
first, addition to the
carbonyl group in a manner analogous to the addition of an alcohol to an
aldehyde or ketone carbonyl group (hemiacetal formation), followed by an elimination reaction, in which water is eliminated
from the previously formed adduct. Addition/elimination is a typical route for substitution
processes in carboxylic acids, esters, amides, and other analgous acyl
compounds in which carbon has the +3 oxidation state.
q
The addition
phase of the reaction is comprised of the first two steps. Essentially an
acid-catalyzed addition of an alcohol to the carbonyl group of the carboxylic
acid. The acid catalyst is shown as the conjugate acid of the alcohol, since in
many cases these esterification (ester-making) reactions are carried out in the
alcohol as a solvent. This is why methyl and ethyl esters are so common.
q
The third
step is just a proton transfer from the –OR’ group to an -OH’ group, so that water can be set up as a good
leaving group.
q
The fourth
step is an SN1-like reaction (with water as the leaving group) in which a carbocationic intermediate
is generated, but the other two resonance structures (especially the second
one) reveal that this is just the conjugate acid of the product ester. Removal of the
proton from this in step 5 generates the ester and regenerates the catalyst.
The last two steps constitute the elimination stage of the reaction.
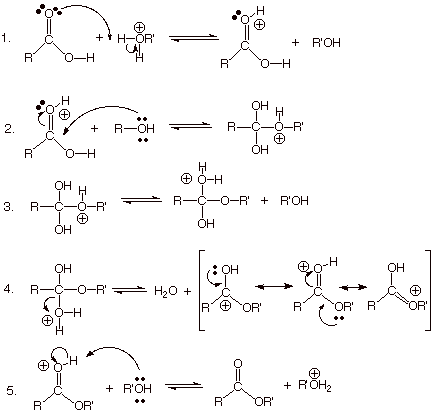
q
Note that
the thermodynamic stabilities of the carboxylic acid and carboxylate ester
functions are very similar, so that the equilibrium constant K is about 1. The use of a large excess
of the alcohol as the solvent, however, can drive the equilibrium to
completion.
q
If the
alcohol is not such that a large excess can be used, the equilibrium can be
driven to the right by the removal of water (the LeChatelier principle) via distillation or the use of a dehydrating
agent.
q
The reason
that these two functionalities are of such similar energy is that the resonance
stabilization of both is about the same. Compare the three resonance structures
below with the three written previously for the carboxylic acid function.
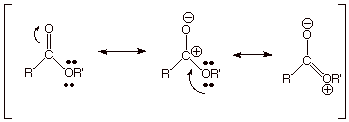
q
It is important
to note that a base-catalyzed mechanism for the esterification of a
carboxylic acid does not exist. When one uses hydroxide anion as a base catalyst (or whatever
other basic catalyst is used), the preferred reaction is not addition to the
carbonyl group, but deprotonation of the carboxyl group to give the carboxylate
conjugate base, which is highly unreactive because of both its resonance
stabilization and its negative charge.
q
In the next
chapter we will see that the reverse reaction, the conversion of an ester to
a carboxylic acid can be carried out in either acidic or basic solution.