CHAPTER 19:ENOLATE ANIONS:NOTES
THIS UNIT DEALS WITH BOTH
ENOLS AND ENOLATES. THE
DISCUSSION OF ENOLS IS IN YOUR
TEXT IN CH. 16 ON PP.577-582.
WE HAVE SEEN THAT THE REACTIVITY OF
CARBONYLCOMPOUNDS (ALDEHYDES AND KETONES) OFTEN FOCUSES UPON ADDITION
TO THE CARBONYL GROUP.HOWEVER, THE PRESENCE OF THIS CARBONYL GROUP CAN
ALSO HIGHLY ACTIVATE NEARBY CARBON-HYDROGEN BONDS (CALLED ALPHA HYDROGENS)
TO UNDERGO VARIOUS SUBSTITUTION REACTIONS. THESE ARE THE REACTIONS WHICH
WE WILL FOCUS ON INTHIS UNIT.
ENOLS.
ENOLS ARE ISOMERS OF ALDEHYDES
OR KETONES IN WHICH ONE ALPHA HYDROGEN HAS BEEN REMOVED AND PLACED
ON THE OXYGEN ATOM OF THE CARBONYL GROUP. THE MOLECULE HAS A C=C AND AN -OH
GROUP, SO IT IS CALLED AN ENE/OL, I.E., AN ENOL.ENOLS CAN BE FORMED ONLY FROM
CARBONYL COMPOUNDS WHICH HAVE ALPHA HYDROGENS. THEY CAN BE FORMED BY ACID
OR BASE CATALYSIS, AND ONCE FORMED ARE HIGHLY REACTIVE TOWARD ELECTROPHILES,
LIKE BROMINE.
- Note that although the carbonyl group is reactive toward nucleophiles at
the carbonyl carbon, it is typically not reactive toward electrophiles, except
at oxygen (not carbon). In contrast, the isomeric enol is reactive toward
electrophiles at carbon.
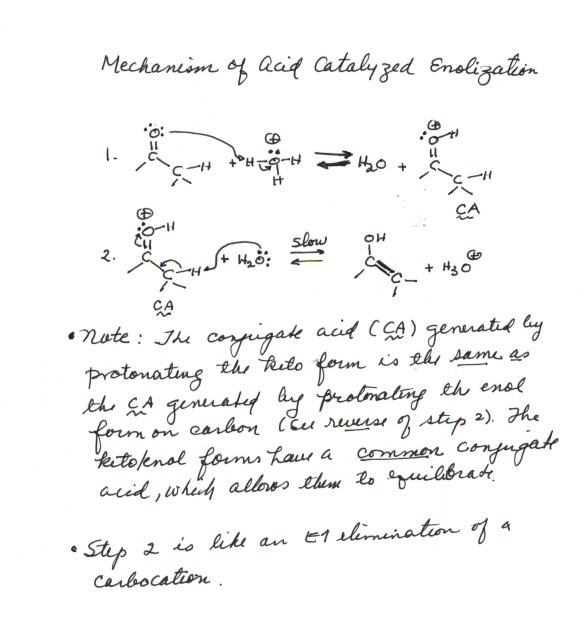
MECHANISM OF ACID CATALYZED ENOLIZATION .
The process of enol formation is called "enolization". It requires
either acid or base catalysis. We consider first the mechanism of the acid
catalyzed process:
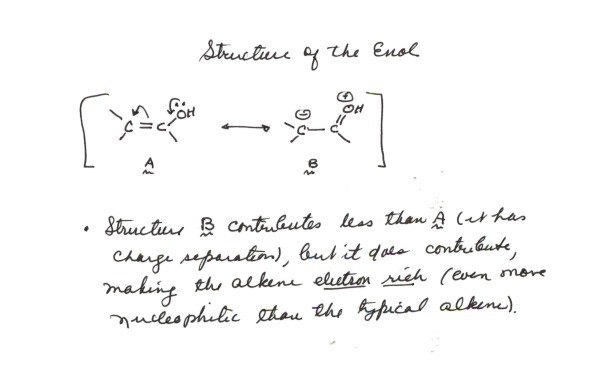
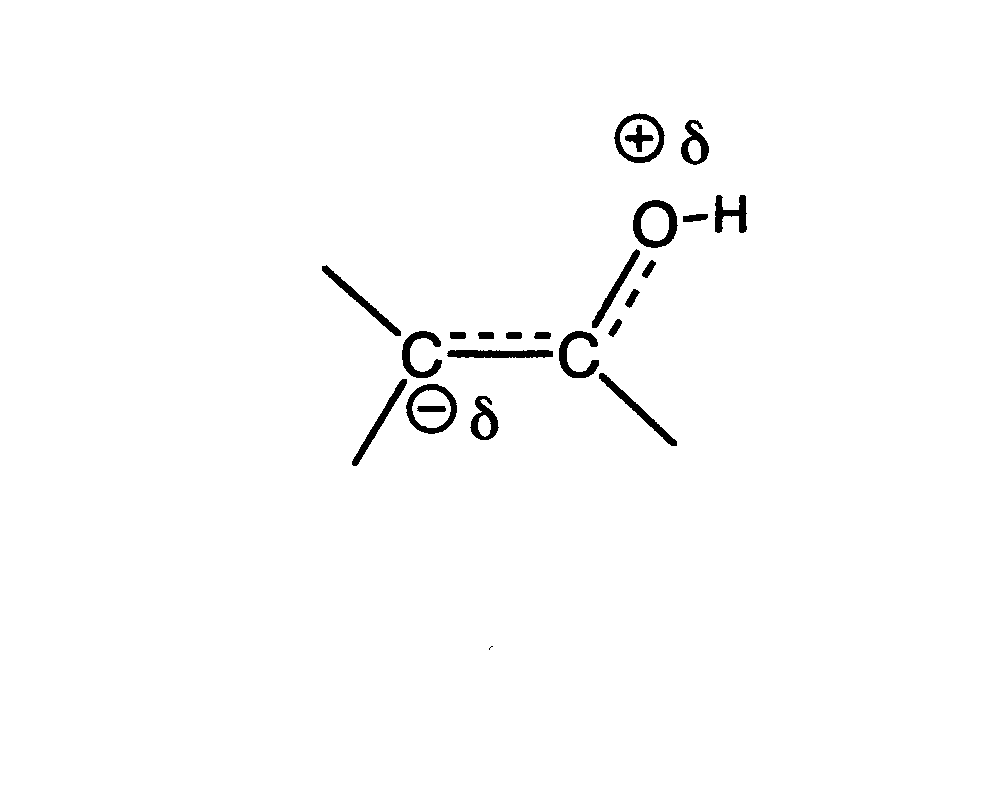
STRUCTURE OF THE ENOL. The
C=C of an enol is very electron rich, because of the hydroxyl substituent,
which can donate an electron pair via the resonance structure shown below.
It is therefore quite nucleophilic, even more so than the typical C=C. It
therefore reacts very rapidly with electrophiles, such as bromine, to result
in overall substitution of Br for H at the alpha carbon atom. The mechanism
for acid catalyzed bromination is given below:
DL/PC Structure of the Enol
RELATIVE STABILITY OF THE ENOL AND KETO TAUTOMERS.
Isomers which differ only in shifting a hydrogen
from one atom to another are often called tautomers. Enols and their
corresponding keto isomers are tautomers. The keto tautomer is typically much
more stable than the enol form, with K's of about 10 to the -5th power. You
should know that this is essentially because the C=O double bond is much more
stable than the C=C double bond.
FORMATION OF BOTH THE ENOL AND ENOLATE UNDER
BASIC CONDITIONS. The formation of an enol under
base catalysis involves the intermediate formation of an enolate, the conjugate
base of the carbonyl compound. So we will first consider the formation of
an enolate, beginning with the dissociation of a carbonyl compound in aqueous
solution to give its conjugate base (that is, we consider the acidity of the
carbonyl compound).
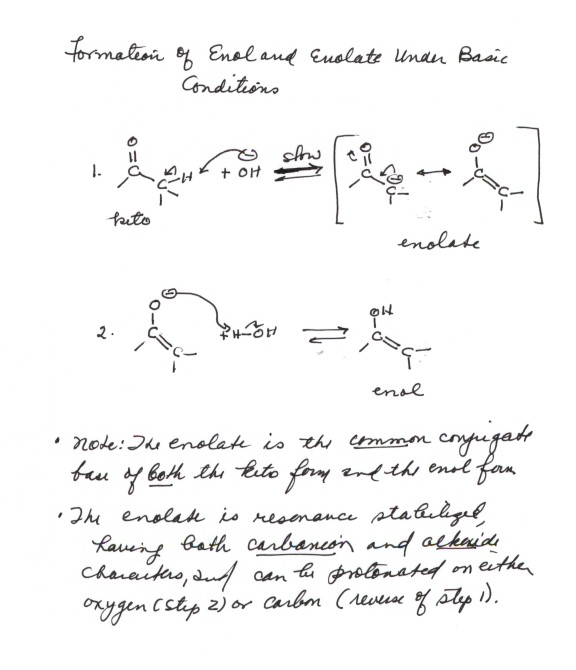
Acidity of Carbonyl Compounds.
In aqueous solution, an aldehyde or ketone which
has an alpha type hydrogen can lose it to water, giving hydronium ion and
the conjugate base of the carbonyl compound, which is called an enolate.
This C-H bond is significantly less acidic than the O-H bond of an alcohol
and much less acidic than the O-H bond of a carboxylic acid. The pK's are
typically about 19-20. Nevertheless, they are outstandingly acidic for H's
bond to carbon. The reason for this is the strong resonance stabilization
of the enolate, which has both carbanion and alkoxide character (see the resonance
structures above). Both resonance structures are comparably stable, so that
the resonance stabilization is large. Although the C=C double bond of the
alkoxide structure is less stable than the C=O of the carbanion structure,
the former has negative charge on oxygen, which is better than having the
negative charge on carbon.
- The Carbonyl group is much more thermodynamically stable
than the alkene group, a factor which tends to cause the canonical structure
having the carbonyl group to be lower in energy than the structure having
the alkene group.
- The canonical structure which has the carbonyl group,
however, has carbanion character, which tends to make it of higher energy
than the structure which has the alkene function, because the latter has alkoxide
character.
- These two opposing factors tend to cancel, making both
structures nearly equal in energy.
- The existence of two nearly equal energy canonical structures
maximizes resonance stabilization.
Base Catalyzed Enolate Formation.
The mechanism for enolate formation in aqueous
base is shown above: This reaction is fast, but the
equilibrium is somewhat unfavorable (the pKa of water is ca. 16, while that
of the ketone is ca.19-20. However, there is easily enough enolate present
to observe efficient reactions since it (the enolate) is a powerful nucleophile.
The Equilibrium between
Ketone and Enolate in Aqueous Base: How to calculate
the position of the equilibrium using a qualitative criterion and a quantitative
criterion; Quantitative generation of the enolate.(Important)
Further, stronger bases can be used to drive
the equilibrium to completion. such as base would be an amide base (LDA, lithium
diisopropylamide, the conjugate base of an amine (pK 38, i.e., about same
as ammonia) . Amide ion (NH2 anion) is basic enough, but it is also nucleophilic
enough to add to the carbonyl carbon, irreversibly. Instead, the more hindered
amide base LDA is used preferentially.
Base Catalyzed Formation of the Enol.
When the enolate is formed, it can abstract a proton
at either oxygen or carbon, both being positions of partial negative charge.
Protonation at oxygen gives the enol, which protonation of carbon yields back
the keto form. Thus, the enolate is the conjugate base of both the keto and
enol forms. any time the enolate is formed in water or a hydroxylic solvent,
it will be in equilibrium with both the enol and the ketone.
Relative Amounts of Enolate and Enol.
Both the enolate and enol are minor components
in equilibrium with the ketone or aldehyde at netural pH. Since the K
for enol formation is larger, there is much more enol than enolate
(see the K values for acid dissociation vs. enol formation). However, in the
presence of strong base, the enol equilibrium is unaffected, but the amount
of enolate increases. So the amount of enolate may easily exceed that of the
enol in basic solutions. In acidic solutions, there will be very little enolate
(it will be protonated to give the enol and keto forms, the neutral forms).
Bromination of the Enol (Acid Catalyzed Bromination).
Both the enol and the enolate provide an opportunity to effect substitution
reactions at the carbon alpha to (attached to) the carbonyl carbon, assuming
that at least one hydrogen atom is attached to this carbon (an alpha hydrogen),
thus permitting enol and enolate formation. In acidic solution, essentially
only the enol is present. Nevertheless, the C=C of the enol is nucleophilic
and reactive toward electrophiles, especially reactive electrophiles like
bromine.The mechanism of this reaction is shown below. Note that the "carbocation"
intermediate, which is involved in this electrophilic reaction is actually
the conjugate acid of the product, which is an alpha bromoketone or aldehyde.
That is, there are two major resonance structures, and the ion has both carbocation
character and oxonium character.The mechanism shown below assumes that the
enol has been formed by the acid catalyzed mechanism already discussed.
Mechanism of the Reaction of Bromine with
an Enol
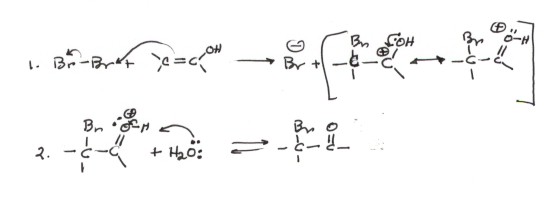
-
Of course, the overall reaction is one which
starts with the aldehyde or ketone and reacts this with bromine to give
the alpha bromoaldehyde or ketone. The first stage of the reaction, the
formation of the enol, is rate determining,i.e., the reaction of the enol
with bromine is very fast. As a consequence, the reaction of an aldehyde
or ketone with chlorine or iodine or other electrophiles occurs at exactly
the same rate as bromination. The rate of enol formation is exactly equal
to the rate of the overall reaction.
- The enolate is even more reactive as a nucleophile than
the enol, and can react not only with strong electrophiles, but even with
much weaker ones, like alkyl halides, to give alkylation at the alpha position.
In Acidic Solution, Enol Formation is Rate
Determining! The subsequent reaction of the enol with bromine is very fast,
so that the enol is prevented from returning to the keto form.
Details
of the Mechanism of Acid Catalyzed Bromination of Carbonyl Compounds.
Mechanism of Base Promoted
Bromination of Carbonyl Compounds.
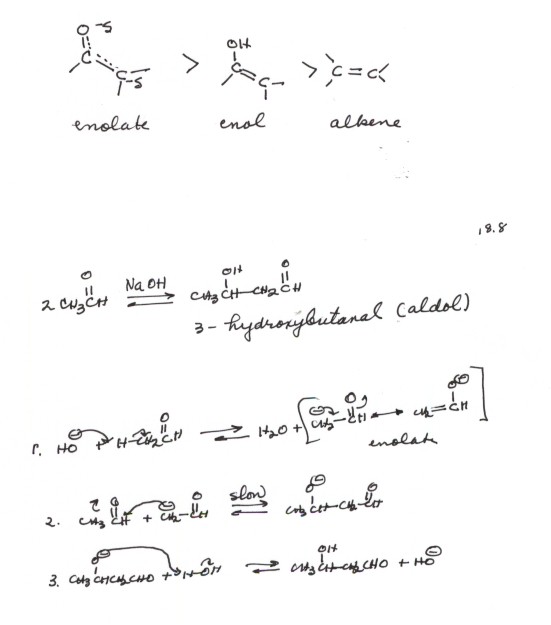
THE ALDOL ADDITION REACTION.
The overall reaction and its mechanism are illustrated for
the simplest aldehyde which undergoes the reaction, ethanal (acetaldehyde).
[Incidentally, why does methanal note undergo the reaction?] The special
importance of the reaction is that it forms a new C-C bond. It does this, in
basic solution,by using the enolate as a nucleophile which adds to the electrophilic
carbonyl carbon. The slow step is the addition to the carbonyl group, as usual.
The product is both an aldehyde and an alcohol (-ol), therefore it was called
an "aldol". The IUPAC name in this particular case is 3-hydroxybutanal.Incidentally,
the reaction also proceeds in acidic solution, using the enol as the nucleophile
and the conjugate acid of the aldehyde as a stronger electrophile.
Mechanism
of the aldol addition:
RELATIVE REACTIVITIES OF THE ENOLATE, ENOL, AND A SIMPLE ALKENE.
Recall that even simple alkenes are relatively nucleophilic
(they react with electrophiles via the pi bond). The enol is more so because
the -OH substituent donates electrons to the pi bond (see resonance structures
for the enol, above). In other words, the enol has some carbanion character
at the carbon beta to the hydroxyl group. The enolate, being negatively charged
, is even more nucleophilic than the enol (please see scheme 18.7). In terms
of resonance structures, the second resonance structure of the enol has charge
separation and is a relatively minor contributor. In the enolate, neither
structure has charge separation and both structures are relatively close together
in energy. One structure has the stronger C=O bond, but the other has negative
charge on oxygen rather than carbon.
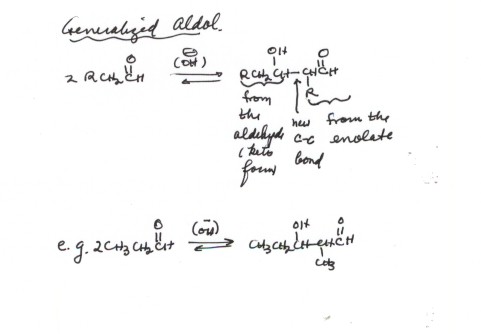
THE ALDOL ADDITION MORE GENERALLY. It is
important to note that an unbranched aldehyde, even a simple one like propanal,
gives a branched aldol, because the enolate or enol always is formed at the
alpha position to the carbonyl group. The branching therefore occurs alpha to
the aldehyde functional group, not alpha to the hydroxyl group of the aldol.
You should be able to predict the structure of an aldol product from any aldehyde
or ketone. As you would expect, the aldol reaction works better with aldehydes
than with ketones, because the equilibrium is less favorable for ketones (recall
the greater thermodynamic stability of the ketone carbonyl). We will see how
this problem can be resolved.
THE ALDOL CONDENSATION REACTION. The somewhat
greater difficulty with which ketones are converted to their corresponding aldol
products can be partially circumvented by carrying out the reaction as an aldol
condensation reaction. In this reaction, in which the conditions are essentially
the same as for the aldol addition, except that the reaction is warmed to RT
or above, the initially formed aldol product is dehydrated to give an alpha,beta
unsaturated carbonyl compound.
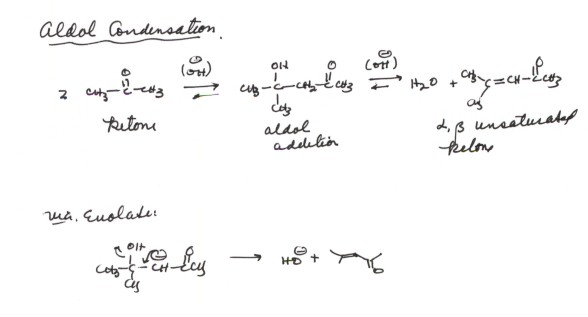
Mechanism of the Aldol
Condensation
- The thermodynamic driving force for the reaction is supplied
by the resonance stabilization of the unsaturated carbonyl function. The alkene
pi bond is in conjugation with the carbonyl group, and the result is a pi
electron system which is delocalized over four atoms, with the resulting resonance
stabilization.
- The mechanism of the elimination reaction is somewhat unusuall,
in that a hydroxide anion is eliminated as the leaving group from the enolate.
Recall, that although it is not a good leaving group, hydroxide anion is a
fairly stable anion. The things which favor its functioning as a leaving group
in this reaction are: (1)There is a unit negative charge already in the molecule
which provides a strong driving force and (2)the reactioni is intramolecular
(favorable entropy). Both of theses are in contrast to simple SN2 reactions
which are intermolecular and there is no negative charge in the alkyl halide.
- Notice that this elimination is stepwise, the base first
abstracting the beta proton to give an enolate, followed by loss of the leaving
hydroxide anion. In contrast, most beta eliminations are concerted. The reason
this one is not is the ability to form a stable enolate intermediate.
- The reaction is called a condensation reaction because
a small molecule (water) is eliminated.
RESONANCE STRUCTURES FOR THE ENONE
THE INTRAMOLECULAR ALDOL CONDENSATION. If two carbonyl
groups are present in the same molecule, the aldol condensation can be carried
out intramolecularly, one carbonyl group providing the source of the enolate
and the other providing the carbonyl function. In most cases only the more stable
5 and 6 memebered rings are formed. See the Scheme below for one example.
Sketch of the Intramolecular
aldol mechanism:
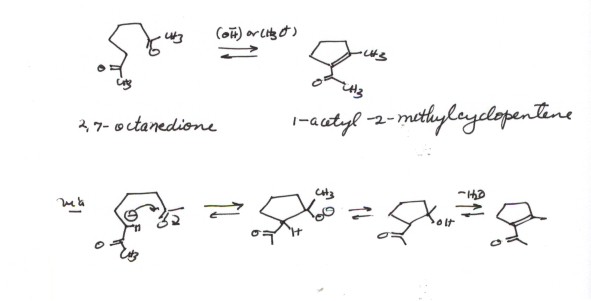
- Notice that the enolate could have been formed by removing
a proton from the methyl group, but in this case addition to the other carbonyl
group would have given a 7 membered ring. Verify this by showing the formation
and ring closure of this enolate.
THE CROSSED ALDOL REACTION.
For a reaction of broader scope, it would be nice to be able
to use two different carbonyl compounds in the aldol, since two different roles
(enolate and carbonyl) are involved. However, if one does this in the most naieve
way, as shown below, four different compounds can result, and generally will
if both compounds have the ability to fulfill both roles.
The Four Products from a crossed aldol reaction between ethanal and propanal
- However, some carbonyl compounds lack alpha hydrogens, and
thus cannot form an enolate or enol. They can therefore only play the role
of the carbonyl function. In such cases, crossed aldol reactions can be successful,
as shown below.
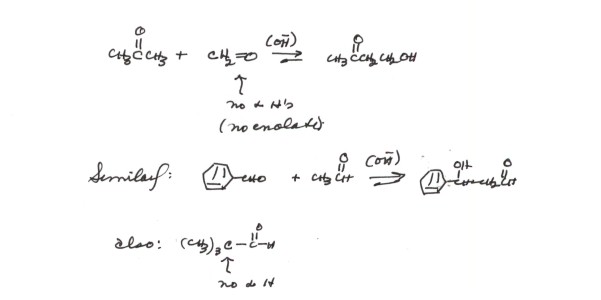
There are two requirements for this procedure
to be effective:
- One of the carbonyl compounds must lack an alpha type hydrogen
and therefore not be able to supply an enolate component. There will therefore
be only one possible enolate.
- The component which does have an alpha type hydrogen must
be added last, gradually, so that a large excess of the non-enolizable carbonyl
component is maintained. The reason for this is that the enolate can still
add to either carbonyl component, giving two different aldols. If an excess
of the non-enolizable component is maintained, the enolate will have a greater
chance to add to this latter component to give the crossed aldol product,
as opposed to the normal un-crossed aldol reaction of the enolizable carbonyl
component.
- In the examples given, an excess of benzaldehyde or methanal
would be maintained, and the enolizable component added last and gradually.
THE
DIRECTED ALDOL REACTION: A MORE GENERAL SOLUTION TO THE PROBLEMS
OF THE NARROW SCOPE OF THE CROSSED ALDOL REACTION IS THE DIRECTED ALDOL.

- This strategy involves the formation of the desired enolate
in a separate step using the strong base LDA (lithium diisopropyl amide) which
is able to convert the aldehyde or ketone quantitatively to its enol. Only
then is the carbonyl component added. Note than different R groups can be
used in the amide, but R= isopropyl is most often used. This gives a hindered
amide which, unlike the simplest amide anion, does not tend to add to the
carbonyl group as a nucleophile, but rather acts exclusively las a base.










