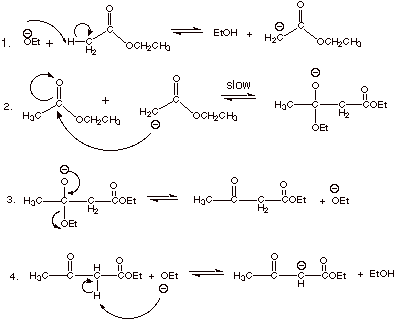
q
NOTE THAT THE ETHOXIDE
BASE IS CONSUMED: THE REACTION IS NOT CATALYTIC, BUT PROMOTED.
q
THE EQUILIBRIUM FOR
STEPS 1-3 IS UNFAVORABLE. WHY? A RESONANCE STABILIZED ESTER FUNCTIONALITY IS
CONVERTED TO A KETONE FUNCTIONALITY.
q
THE THERMODYNAMIC
DRIVING FORCE FOR THE REACTION IS PROVIDED BY THE HIGHLY EXERGONIC
NEUTRALIZATION REACTION OF STEP 4.
q
THE BETA KETO ESTER
PRODUCT, ETHYL ACETOACETATE, IS OBTAIN UPON ACIDIFICATION IN THE WORK-UP.