CONTINUATION OF SUBSTITUTION;
ELIMINATION
Solvent Effects upon SN1
and SN2 Reactions.
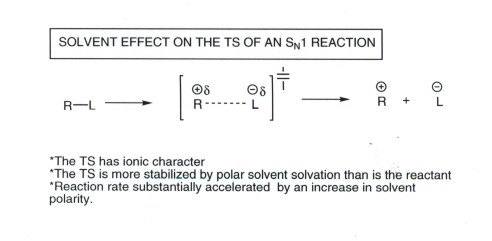
The transition state of the rate determining step for the SN1
reaction has developed ionic character and is well on the way (see Hammond
Postulate)to forming a carbocation and a leaving group anion. Thus, the TS has
carbocation and anionic character. Since ions benefit greatly from solvation
by polar solvents, it is clear that an increase in the solvent polarity will
yield an increased stabilization of the TS (compared to the energy of the reactant
halide, which is moderately polar, but not ionic) , thus greatly increasing
the rate. Thus, SN1 reactions occur especially rapidly in polar
solvents like water, methanol, ethanol, or acetic acid.
Solvent Effects upon SN1
Reactions.
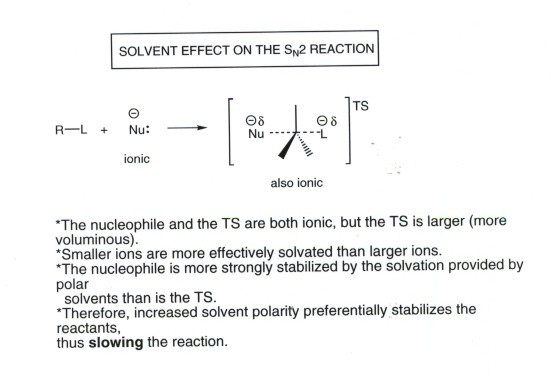
The situation with SN2 reactions is
more complex. In the familiar type of SN2 reaction in which the nucleophile
(a reactant) is negatively charged, solvation of the nucleophile by a
polar solvent would tend to greatly slow the reaction rate (stabilization
of the reactants increases the activation energy). However, the TS is also anionic,
and is similarly stabilized by a polar solvent. So the net effect is not so
large as in the SN1 case, but there is still a significant effect
in the direction of slowing the reaction in a more polar solvent. This must
mean that the reactant anion is more stabilized by the solvent than is the
TS. This is fundamentally because the TS is a much larger anion, with the
negative charge smeared out over a greater volume. The larger an ion, in general,
the less efficiently it is solvated. Nevertheless, it is important to note
that in such SN2 reactions an at least relatively polar solvent is
required in order to dissolve the anionic nucleophile (as a salt).
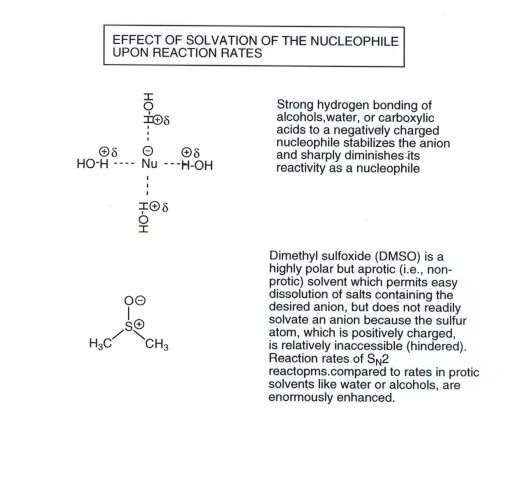
SOLVENT EFFECTS UPON NUCLEOPHILICITY
- One particular effect of solvent polarity, viz., hydrogen
bonding ability, has an especially powerful effect upon the strength of
a nucleophile and therefore upon the rate of an SN2 reaction.
- A negatively charged nucleophile can strongly hydrogen
bond to a protic solvent, i.e., a solvent having a relatively acidic
proton. This H-bonding strongly stabilizes the nuceophile and lowers its
reactivity. Hydrogen bonding solvents include water, alcohols, acetic
acid, etc.
- However, to perform an SN2 reaction using an
anionic nucleophile, the salt which provides the anion must dissolve. This
requires a relatively polar solvent. What to do??
- The dilemma is solved by using what is called a "dipolar
aprotic solvent", i.e., a solvent which is polar enough to dissolve
the salt well, but does not have an acidic hydrogen to stabilize
the nucleophile. Such solvents include DMSO (dimethyl sulfoxide) and DMF (dimethyl
formamide).
- The rate of an SN2 reaction in DMSO is often
enhanced by a factor of as much as a million-fold.
DMSO is a polar solvent because the S-O bond is highly
polar, very close to ionic, because to form a pi bond with oxygen (i.e.
a double bond) S would have to use a very high energy d orbital. Such bonding
is relatively weak.
NUCLEOPHILIC POWER (NUCLEOPHILICITY).
We have seen that the potency of a given anionic nucleophile
can be sharply increased by assuring that the anion is not stabilized by hydrogen
bonding (using a dipolar, aprotic solvent). For a series of different nucleophiles,
obviously, the potency as a nucleophile, which would be measured by the relative
rates of reaction with a standard alkyl halide like methyl iodide, can also
vary quite widely. The nucleophilicity depends on a number of different factors,
including:
- Stability of the anion. This is an inverse dependence,
since the more stable an anion is , the less reactive it is. Anion stability
can be assessed in terms of the basicity of the anion, i.e., its reactivity
toward a proton. The more basic an anion is, the more reactive it is in general.
- For example, the hydroxide ion is more basic than an acetate
anion (i.e., water is less acidic than acetic acid, these being the conjugate
acids of the bases being considered). Therefore, hydroxide ion is more nucleophilic
than the acetate ion. Please note that basicity is reactivity toward a proton,
while nucleophilicity in the present context refers to reactivity toward carbon.
The two kinds of reactivity tend to parallel each other, with exceptions to
be discussed further on.
- We could include a neutral nucleophile like water or methanol
in the foregoing comparison. Since water or methanol is less basic than even
acetate anion, these former molecules are still less nucleophilic than acetate
anion. The sequence of nucleophilic reactivity is therefore water,methanol>acetate
anion>hydroxide anion.
Polarizability of the Nucleophile at the Reactive Atom
of the Nucleophile.
- The basicity of the anion is the main factor controlling
nucleophilicity so long as the comparison involves only nucleophiles with
a common atom as the reactive site. Thus, water, methanol, acetate ion,
and hydroxide ion are all nucleophiles which react at oxygen. In such cases,
nucleophilicity parallels basicity quite closely.
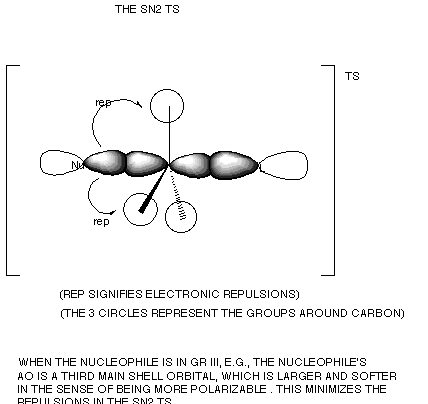
- However, when comparisons include nucleophiles which react
at different atoms, dramatic deviations from the correlation of nucleophilicity
and basicity are found. For example, bromide ion is far less basic than
hydroxide ion, but is substantially more nucleophilic, and chloride ion
is at least comparable nucleophilic to hydroxide ion. Nucleophiles which react
at halogen, sulfur , phosphorus, or other heavier atoms are typically much
more reactive than oxygen-centered nucleophiles. For example, HS- is far more
nucleophilic than hydroxide ion, though the sulfur based anion is less basic
than hydroxide ion. This is because the electrons around heavier atoms are
farther from the nucleus and are less tightly held; therefore "softer"
in the sense that they distort more easily and are able to minimize the steric
repulsions to which the SN2 TS is so sensitive.
- This effect is often referred to as polarizability.
ELIMINATIONS
- As we have previously seen, elimination (E) reactions
are those in which a single molecule is split into two or more molecules.
Most often a small molecule like water or HCl is eliminated from the larger
starting material; hence the term elimination.
- The most common elimination mechanism is the so-called
E2 elimination. This designation refers to the circumstance that the rate
determining step (and the only step) is bimolecular. The reaction is
concerted (see below).
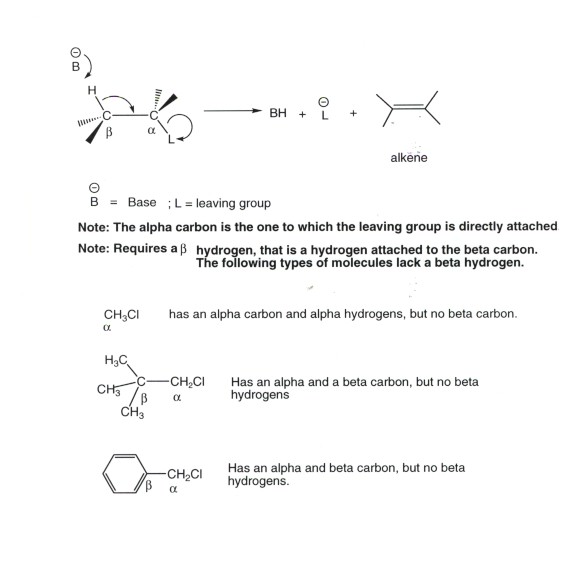
- The most common structural format for any mechanistic type
of elimination is what is termed the b elimination,
in which a proton which is beta to a leaving group is removed by a base.
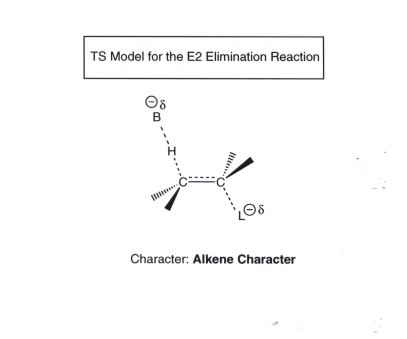
- The reaction produces alkenes.
Since the reaction is concerted, the TS has alkene character (derived from
reactant character). When two non-equivalent beta protons are available
to generate different alkenes, the TS which has the more stable alkene character
is at least somewhat (but not highly)favored. That is to say the reaction
is regioselective but not highly regioselective. Recall that the greater the
degree of substitution, the more stable the alkene.
- A base is needed to pull off
the beta proton. In order to have a fast rate of reaction under mild temperature
conditions, it is usual to use a strong base such a hydroxide ion,
or more commonly, ethoxide ion. The latter is obtained from ethanol by reaction
with sodium metal.
THE E2 REACTION
THE
TS MODEL FOR THE E2 REACTION:
REGIOCHEMISTRY OF E2 ELIMINATIONS.
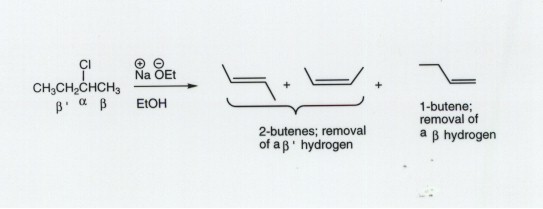
- In some cases, there may be two different (i.e., non-equivalent)
types of beta hydrogen leading to two (or more) different alkenes. In this
case we use the Method of Competing Transition States to predict which
will be the major product.
- It is helpful to label the two different beta hydrogen types
as beta and beta prime. In the example given below 2-chlorobutane gives both
1-butene and the 2-butenes (cis and trans). The latter are the major products
and the former is minor.
- In general, such E2 eliminations lead to both (or all of
the possible) products, but the one is favored which is the more highly
substituted and therefore more stable alkene. This is called the Saytzeff
Rule.
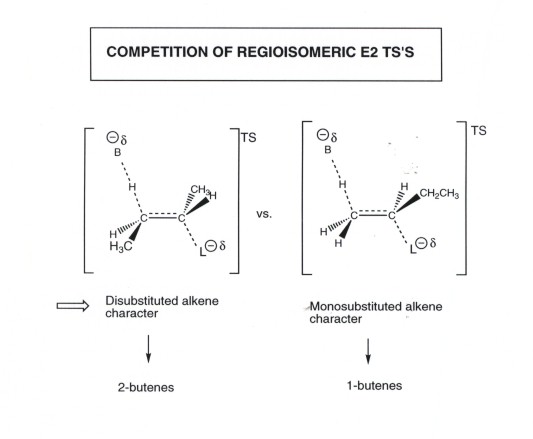
- In the case of our example, 2-butenes are disubstituted
alkenes and 1-butene is a monosubstituted alkene.
- The rationalization uses the two TS models and argues that
disubstituted alkene character in the TS is more favorable than monosubstituted
alkene character.
- The selectivity for the more highly substituted alkene is
generally not very strong. First, the energy difference is relatively small
even between the alkenes (2.7 kcal/mol) and second because the reaction is
at least somewhat exothermic (as a successful concerted reaction must be),
so the TS doesn't have extensive alkene character.
COMPETING REGIOCHEMISTRIES
IN E2 REACTIONS
METHOD OF COMPETING
TRANSITION STATES
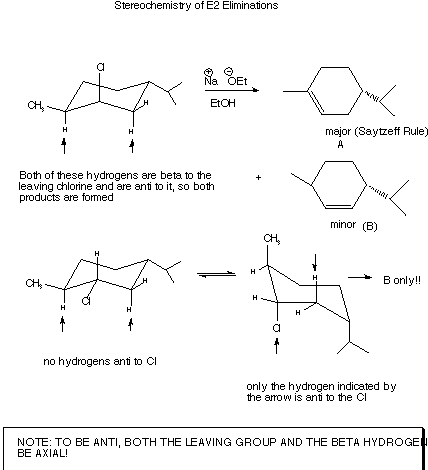
STEREOCHEMISTRY OF E2 ELIMINATIONS.
- In order for the pi bond of the intended alkene product
to develop, the TS must have strong pi bonding. This essentially requires
that the 2p orbitals which develop on the alpha and beta carbons be parallel.
In turn, this engenders the need for the beta proton to be anti to
the leaving group. No other relationship (gauche, in particular) will develop
as efficient pi bonding between these AO's. Note also, that if the dihedral
angle theta between the orbitals is zero degrees, pi overlap is still strong,
but the conformation is eclipsed and disfavored by torsional effects.
Only the 180 and 0 degree dihedral angles provide for efficient pi overlap.
- In acyclic systems, it is no problem to generate the required
conformation with the beta hydrogen anti to the leaving group, but in the
more rigid cyclic series it may be impossible. The requirement of anti beta
hydrogen and leaving groups is especially well met on a cyclohexane ring when
these two groups are both axial, as illustrated in the scheme below.
COMPETITION BETWEEN THE
E2 AND SN2 REACTIONS
- Since a base is also a nucleophile (the requirements
for both are an unshared pair of electrons) in any given situation
in which a beta hydrogen is present, either or both of these two reacitons
may occur. Frequently, they are competitive.
- If the base is much stronger as a nucleophile than as
a base, such as a sulfur or halide type anions, the SN2
reaction will naturally dominate.
- If the leaving group is attached to a primary carbon,
the SN2 will predominate any way , because of the great
rapidity of this mechanism at a primary carbon. However, secondary or tertiary
systems usually lead to predominant elimination.
- If no beta hydrogen is present, the E2 is unable to occur,
so that the SN2 would dominate.









