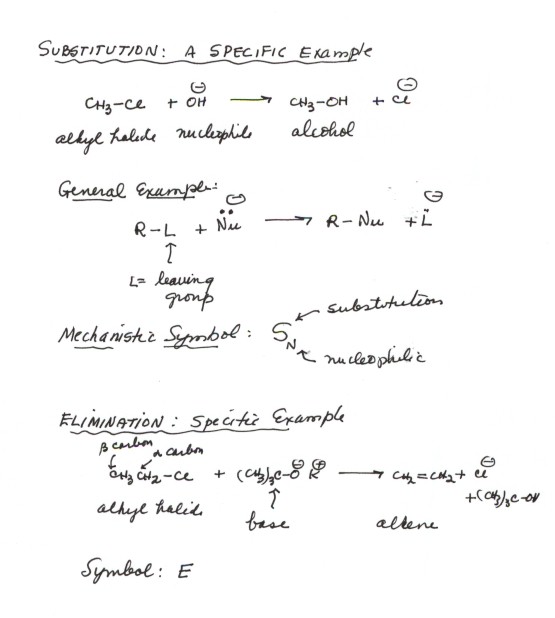
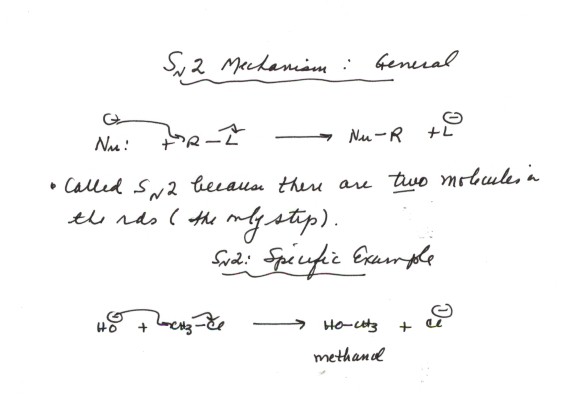
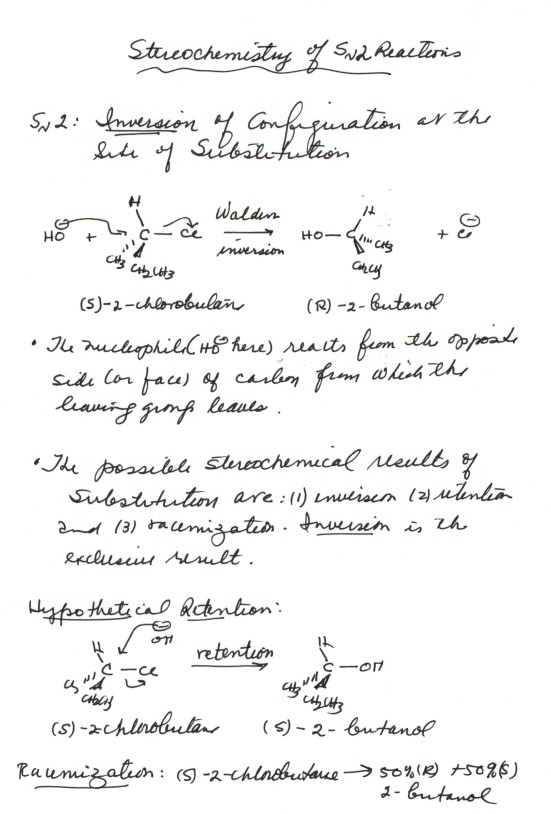
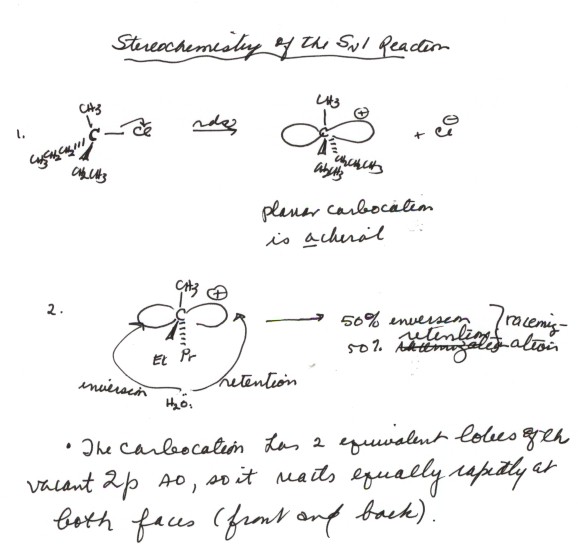
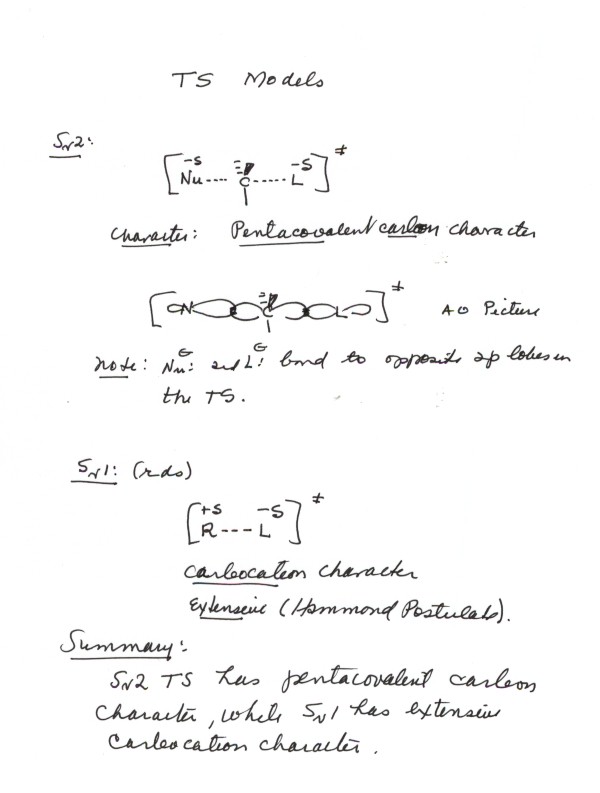
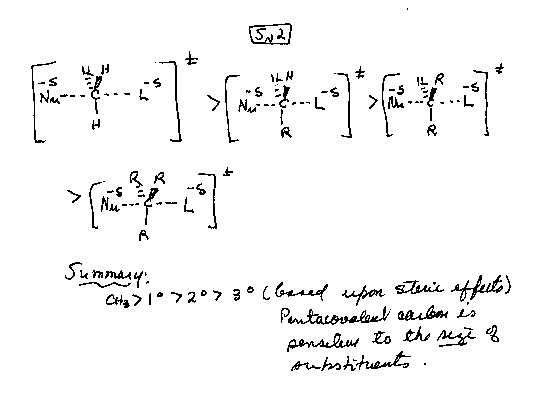
-
As a result of the opposing rate tendencies
in the two mechanisms, it turns out that tertiary systems, which
are the fastest at the SN1 and slowest at the SN2,
virtually always can be relied upon to undergo reaction via the SN1
mechanism, i.e., via a carbocation mechanism.
-
-
In contrast, methyl and primary
systems which are good at SN2 and slow at SN1 virtually
always react via the SN2 mechanism.
-
-
Secondary systems are more difficult
to predict, but the main factor here is the strength of the nucleophile.
Remember that the nucleophile is present in the SN2 transition
state but not in the SN1 TS. The stronger the nucleophile,
the faster the SN2 reaction, but increasing the nucleophilic
strength has no effect on the SN1 reaction. So, with weak
nucleophiles like water or methanol, secondary systems prefer the SN1
mechanism, but with strong ones like hydroxide or other negatively charged
ions they will proceed via an SN2 mechanism.