Chapter 1: Molecular Orbital
Concepts
A. Concepts of MO Theory.
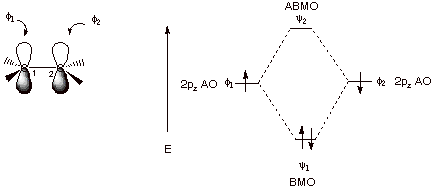
1. Strong Covalent Bonds. Consider the pi bond of ethene in simple molecular orbital terms (The qualitative results would be the same for any pi or sigma bond.
q
The overlap of the two atomic
orbitals (AO’s) results in the formation of two molecular orbitals
(MO’s), one of which is lower in energy than the original AO’s (the
bonding MO or BMO) and the other higher in energy than the original AO’s
(the antibonding MO or ABMO).
q
Two paired electrons can
occupy the lower energy MO, resulting in pi bond formation.
q
The fundamental
requirements for strong bond formation are:
o
Efficient overlap
o
Two paired electrons.
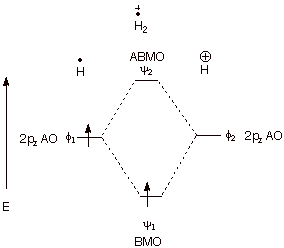
2. Weak Covalent Bonding.
a. One electron bonding. If only one electron is present, it can occupy the
BMO, also resulting in significant bonding, but since there is only one
electron to contribute to the bonding, the bonding is not nearly as strong as
in the case of two electron bonding.
q
The hydrogen molecule
positive ion. Consider the combination of a proton and a hydrogen atom. This
results in the formation of H2 with just one electron (it is a
cation radical). The bonding energy of this species is actually fairly
impressive, being measured as 64 kcal/mol.
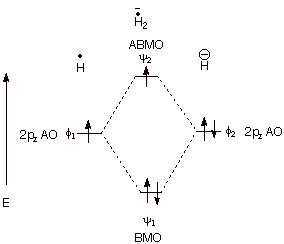
b. Three Electron Bonding. Consider the combination of a hydrogen atom (one
electron) with a hydride anion (two electrons). In this case the third electron
must go into the ABMO, thus negating some of the bonding energy derived from
the BMO. However, there are two electrons contributing to bonding, and just one
electron to antibonding. Consequently, there is net, albeit, relatively weak
three electron bonding.
q
Three electron bonding
is an important means of stabilizing some radical species. Consider the
methoxymethyl radical shown below. A 2pz AO on carbon (the radical
site) overlaps with a 2pz AO on oxygen which contains an unshared
pair of electrons. Overlap between the two 2pz AO’s results in
the formation of MO’s, which are populated by three electrons, as shown.
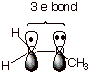
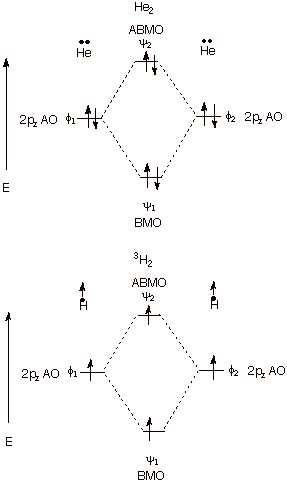
3. Zero Net Bonding. When there are zero or four electrons present or when
there are two electrons which are unpaired, no net bonding results. Since the
energy we are discussing is electronic energy, when there are no electrons
present, there is obviously no bonding energy. The four electron no-bond case
is nicely illustrated by dihelium, which has almost exactly zero bonding. The
bonding provided by two electrons in the BMO is cancelled to an excellent
approximation by the antibonding engendered by two electrons in the ABMO. The
case of two unpaired electrons is illustrated by the triplet state of
dihydrogen. Both the dihelium (4 e) and dihydrogen triplet (two unpaired
electrons) cases are illustrated below. In the latter case, the second electron
cannot enter the BMO because off Pauli Exclusion.
B. The MO’s and
Their Energies.
We
want to have a slightly closer look at the pi MO’s of ethene and their
energies. The approximation we use is an extremely simplified one known as the
Huckel MO or HMO approach. The approach neglects the sigma electrons,
considering only the pi electrons , and approximates the MO’s as linear
combinations of the atomic orbitals (the LCAO approximation).
1. The LCAO-MO
Approximation for the Pi System of Ethene.
The pi MO’s of ethene are assumed to have the
somewhat general form given below, where the a’s represent the numerical
coefficients of the two AO’s. Since there are two MO’s, the
subscript i is used to write a
general form for both of these MO’s (where i is 1 for the BMO and 2 for
the ABMO (i.e., numbering from the MO of least energy to the highest). The
coefficients have two subscripts, since potentially each atom may have a
different coefficient in each MO. Thus a11 is the coefficient of
atom 1 in MO 1, a12 is the coefficient of atom two in MO1, a21
is the coefficient of atom 1 in MO 2, etc. Essentially, theses coefficients, aij,
give the weight of the 2pz AO of atom 1 or 2 in MO i.
![]()
The
relative magnitudes of the coefficients can be found from symmetry arguments (they
must have the same absolute magnitude, i.e., equal or opposite, because C1 is
equivalent to C2) or by Variationally minimizing the energy with respect to the
coefficients (not considered here). The absolute values of the coefficients are
found by imposing the normalization condition, i.e., when integrated the square
of the wave function gives the value one, corresponding to a total electron
density of one. Using symmetry, we can see that one of the MO’s (the BMO)
has equal coefficients (here set to 1) and the other opposite coefficients (set
to 1 and –1). These are shown as “primed” wave functions.
When normalized (subject also to the condition of the neglect of the overlap
integral, which is usually employed in the HMO method), the final form of the MO’s
is obtained (shown as unprimed wave functions).
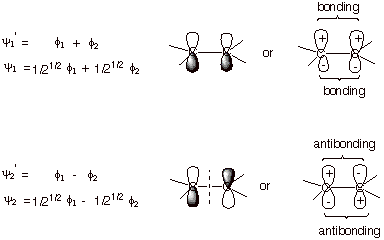
q
Note that in the BMO,
the bonding corresponds to positive overlap on both faces of the molecule,
while in the ABMO, antibonding results from negative overlap. Positive overlap
occurs when both overlapping lobes have the same sign (both plus or both
minus). As a result, the MO accrues additional electron density in the region
between the two carbon atoms, which corresponds to the main bonding region. In
the ABMO, the subtraction of the AO’s results in less electron density in
the bonding region.
2. Energies of the
MO’s.
The
energies of the MO’s can be obtained by solving the HMO secular
determinant. In the HMO approximation, they are expressed in terms of the
parameters a and b. The former is
called the coulomb integral and roughly corresponds to the energy of an
electron in the 2p AO of one of the carbons (no covalent bonding). The second
parameters is called a resonance integral, and corresponds to the stabilization
resulting from overlap and bonding (covalent pi bonding). An energy level
diagram of these orbitals and their energy is illustrated below.
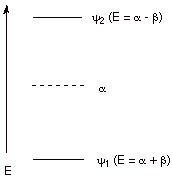
q
Since both parameters
are negative (they correspond to stabilization or lowering in energy), the plus
sign corresponds to the BMO and the minus sign to the ABMO.
q
The pi bonding for each
electron in ethene is thus b, so that for the two electorn bond the molecular
bonding energy is 2b.
q
The a part of the energy
corresponds to the stabilization the electron would have on the atom itself,
without covalent bonding or overlap, so although it contributes to the atomic
binding (the binding of the electron to its nucleus), it doesn’t
contribute to the covalent bonding in the pi bond.
C. Antibonding MO’s.
In
ethene, only the BMO is occupied; the ABMO is vacant. It contributes nothing to
the energy of the system. It is sometimes described as a virtual MO. The
question sometimes arises of what use is the concept of an ABMO or of any
virtual orbital. Essentially it is that the presence of this MO reminds us of
the ability of the molecule to accept an electron into this orbital in the
context of a reaction. There are
two principal ways in which the ABMO ceases to be a virtual orbital and becomes
a real, occupied MO.
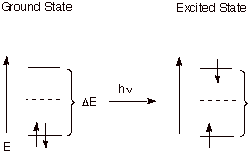
1. Excited States.
When a molecule absorbs radiation which satisfies the Einstein equation dE = hn, it promotes an electron from the BMO to the ABMO. This excited state of the original molecule is highly energetic and capable of undergoing potentially useful chemistry (photochemistry) which the more stable ground state is incapable of.
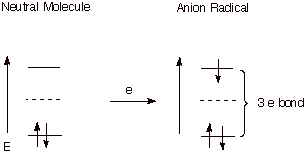
2. Anion Radicals
In
the context of purely thermal chemistry, as opposed to photochemistry,
electrons may be transferred from an electron rich species ( called a single
electron donor) to a neutral molecule to form a negatively charged species
which also has an unpaired electron in the ABMO. These species are called anion
radicals. Again, they have an electron in a relatively high energy antibonding
orbital, so they are much more reactive, in general than the original neutral
molecule. They, in fact, are excellent representatives of species having a three
electron bond. Since the electron in
the antibonding orbital diminishes the pi bonding present in the molecule, the
species is highly reactive.
Electron donors suitable for transferring electrons to ABMO’s of
neutral molecules include alkali metals, negative electrodes, and some electron
rich organic molecules.
The
energy required to introduce an electron into the ABMO of a neutral molecule in
solution is called the reduction potential. The higher the energy of the ABMO into which the electron must be
introduced, the more negative the potential needed for the electron transfer.
Reduction potentials as typically measured are relative, not absolute, and are
typically referred to the saturated calomel electrode (SCE). In general, two
structural factors are especially important in lowering the energy of the
appropriate ABMO and thus the reduction potential of a substrate molecule
(i.e., making it easier to reduce).
First, the more extended the conjugated system, the lower the energy of
the ABMO and the more easily reducible the substrate. Second, pi bonds which
have strongly electron-withdrawing groups attached, which can stabilize the
negative charge of the anion radical, also have lower energy ABMO’s. One
of the more easily reducible organic substrates is tetracyanoethylene. The
anion radical of this substrate is so stable that its salts can be isolated. A
detailed single crystal neutron diffraction study of one such salt reveals the
distribution of the odd electron and thus the shape of the ABMO. Interestingly,
the AO’s on the central carbons are bent back, away from one another, in
order to minimize overlap, which is negative, antibonding overlap. The
knowledge of theshapes of ABMO’s comes in handy in several connections
which we will be considering further on. Incidentally, the MO which has the
single electron is often referred to as the SOMO (singly occupied MO). This
orbital is especially important because it determines where the odd electron is
and also, largely, the negative charge.
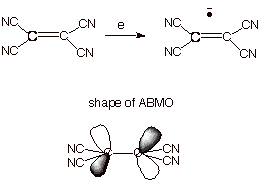
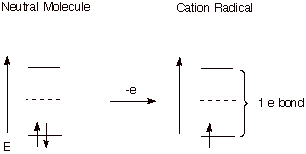
D. Cation Radicals and One
Electron Bonds.
Cation
radicals are reactive organic species which can be formed by removing an
electron from an occupied MO of a neutral molecule. The pi bond in such a
system is an excellent example of a one electron bond. Nevertheless, these
species are highly reactive, since they do not have the typical strong two
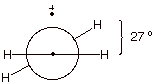
electron bond. The weakening of the pi bond in the ethene cation radical is
reflected in the structure of this species which, unlike ethene, is twisted by
ca. 27o out of planarity.
E. Allylic conjugation.
1. MO’s of the Allyl
System (Radical, Cation, and Anion).
The
allyl system is a three carbon system in which the carbon atoms are all sp2
hybridized, and the trigonal planes are all coincident, so that the
unhybridized 2pzAO’s are all “parallel” and
undergoing efficient pi overlap. Since all three atoms are involved
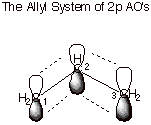
in the pi overlapping, the
MO’s are delocalized over three atoms, rather than the more usual two
atoms as exemplified in the pi bond of ethene. A conjugated pi system is one
which, like the allyl system, has the electrons shared by more than two atoms.
We will not invoke the mathematical coefficients of the MO’s, but will
use the simple qualitative depiction of them shown below in order to understand
the electron distribution in the MO’s and the energies of the
MO’s.
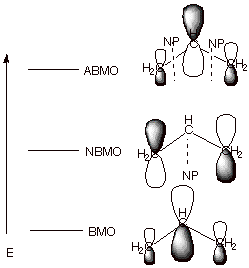
q
First, since there were
now three 2pz AO’s in the basis set, there are three
MO’s which result from their combination by overlap. Only one MO is
bonding, and there is an antibonding and a nonbonding MO. A nonbonding MO
(NBMO) is one which has the same energy as the basis set AO, i.e., has not
benefit from delocalization.
q
The BMO has a larger
coefficient at C2 (the central carbon) than at C1 and C3, corresponding to a
larger electron density at C2 in this particular orbital. Note also that all
overlaps are positive and bonding, corresponding to a relatively lower energy
MO.
q
The NBMO, in contrast,
has the largest coefficients at C1 and C32 (the terminal atoms), and a zero
coefficient at C2 (there is a nodal plane, NP, through C2, where the coefficient changes sign from positive
to negative. Note that there is no overlap between neighboring atoms, e.g.,
C1-C2 or C2-C3, because the coefficient at C2 is zero (zero electron density).
This corresponds to the circumstance that this MO is nonbonding.
q
The ABMO returns to the
general shape of the BMO, in the sense that the largest coefficient is at the
central atom. The high energy of this MO corresponds to the circumstance that
all neighboring group overlaps are negative and antibonding.
q
Note that, the most
bonding MO in a conjugated system is always fully bonding, i.e., has no
negative overlaps and no nodal planes. The next MO in terms of increasing
energy has one nodal plane, and so on. The energy increases monotonically with
the number of NP’s because these correspond to nonbonding or antibonding
interactions.
2. The Pi Bonding
Energy and Delocalization Energy
of the Allyl Carbocation.
The
allyl carbocation has 2 pi electrons, as may be seen by examining a canonical
structure of the ion. The double bond of the latter contributes 2 pi electrons,
while the
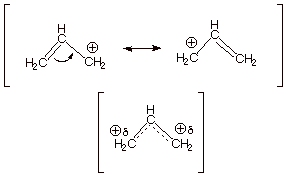
carbocation center has a
vacant 2pz AO and contributes zero electrons. The energy level
diagram is therefore as shown below.
Note that the total pi electron energy is more negative (more bonding)
than the two electrons of the ethene pi bond, by 0.82b. This is the
delocalization energy, i.e., the additional stabilization of these two
electrons derived from their delocalization over three atoms, instead of just
two. Formally, the key to the lowering of the energy is the lower energy of the
BMO in the allyl system than in the ethene system.
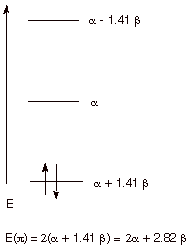
We might also note, that since the BMO is heavily concentrated on the central carbon atom, most of the electron density of the allyl cation is located at C2. Therefore, the positive charge is at C1 and C3as typical resonance structures of the allyl cation indicate. If one calculates the electron densities, it is found that, in precise agreement with the resonance theory, there is exactly zero positive charge on the central carbon and exactly one-half unit of positive charge on each of the terminal atoms. As shown in the diagram below, the contribution to the electron density at a specific atom from a single electron in the BMO is given by the square of the coefficient of that atom in the BMO. For atoms 1 and 3, that is 1/4; for atom 2 it is 1/2. Since there are two electrons in
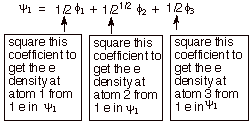
this MO, we must double this, giving 1/2 for C1 and C3 and 1.0 for C2. To be neutral, each carbon should have exactly 1.0 2pz electrons, so the net charge at C2 is zero, and at C1 and C3 it is +1/2. We can formalize this procedure as follows, where q is the total pi electron density at atom i , Qi is the charge density at atom i (1-qi), Ni is the number of electrons in a given orbital (the occupancy number), aij is the coefficient of atom i in the jth MO, and the summation is over all singly or double occupied orbitals (since each occupied MO contributes something to the electron density). In the simple case of the allyl cation, there is only one occupied orbital, so no summation is required.
![]()
As an exercise, use this formala to calculate the charge densities at C1, C2, and C3 in the allyl cation, radical, and anion. The radical, as we will see, has 3 electrons, and the anion has 4. As a further exercise, see if you can calculate the spin distribution in the radical, assuming that all of the electron spin comes from the singly occupied MO (the SOMO). Then compare these results with the results of applying simple resonance theory to the cation , radical, and anion.
F. Conjugated Dienes.
1. Energy Levels and Delocalization Energy of
1,3-Butadiene.
The simplest conjugated diene, 1,3-butadiene, has a conjugated system in which the pi electrons are delocalized over four carbon atoms. The energy levels are show in the diagram below. Since there are four basis set AO’s, there are four MO’s, two of them bonding and two antibonding. Note the symmetrical disposition of the MO’s about the nonbonding level. As an exercise, fill in the appropriate number of electrons and calculate the pi electron energy and delocalization energy of this system. The appropriate comparison, for the purposes of calculating the DE is with two ethene molecules.
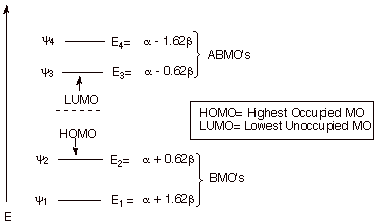
2. Electron Densities, and Charge Densities in
Butadiene.
As an exercise, use the previously given formula to calculate the qi’s and Qi’s for neutral butadiene. By symmetry, of course, the results for C1 and C4 are identical, as are those for C2 and C3. Are the results physically reasonable?
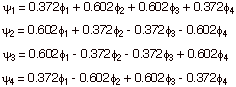
Note that not only are the energies of the first and fourth MO’s related by symmetry, these MO’s have coefficients which are identical in absolute values. Similarly, the coefficients of the second and third MO’s are closely related. Again, also note that the first MO has zero nodes, the second has one, the third two, and the fourth three.
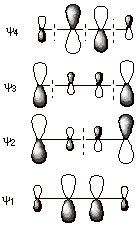
3. Ion Radicals of Butadiene.
The cation radical of butadiene is formed when one electron is removed from neutral butadiene. As an exercise, construct the energy level diagram of butadiene cation radical. Assuming that all of the charge and spin arise from the singly occupied MO (SOMO; a good approximation), calculate the charge(Qi) and spin (rI) at each carbon atom (again, you may use symmetry considerations, i.e., C1 = C4 and C2 = C3)). Do the same for the butadiene anion radical, which has one additional electron (five) compared to neutral butadiene. Compare the charge and spin densities in the anion and cation radicals. If the anion radical were to be protonated, where would it be likely to react? Write an equation for this reaction. Similarly, if the cation radical were to react with a nucleophile such as hydroxide ion, where would it react. Draw the equation for this reaction, also.
G. Longer, Acyclic Conjugated Systems; Generalizations
1. Even Number of Carbon Atoms
The next higher conjugated system having an even number of carbon atoms in it would be a conjugated triene, such as 1,3,5-hexatriene. You should anticipate by now that with six carbon atoms there would be six pi MO’s, three of which would be bonding and three antibonding. The antiboding MO’s would each be paired with a BMO, in terms of symmetry about the nonbonding level. The number of nodes would increase in the sequence 0,1,2,3,4,5 in order of the increasing energies of the MO’s. Since the LUMO of butadiene was considerably lower in energy than that of ethene, you could also expect that a continued increase in the length of the conjugated system would continue to drop the energy of the triene LUMO and correspondingly increase the energy of its paired HOMO.

As an exercise, draw out the energy level diagram for a triene system and compare it with the corresponding diene system.
2. Odd Number of Carbon Atoms.
Just as the allyl system had an odd number of carbon atoms (three) in the conjugated system, there are also conjugated systems having 5,7, etc. carbon atoms in them. The pentadienyl system is the simplest of these. The main difference between these
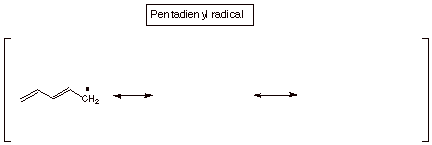
odd carbon systems and the even carbon ones is that the odd carbon systems (which can have, like the allyl system, a carbocation, a radical, and an anion) have a NBMO. Like the allyl radical, the pentadienyl radical has its odd electron in the NBMO, so we only need to know the coefficients of the NBMO in order to predict the odd electron density in this radical, or for that matter the positive charge density in the carbocation and the negative charge density in the carbanion. Incidentally, this latter circumstance may be somewhat non-intuitive. The radical, which has 1 electron in the NBMO is, as a hydrocarbon radical would be expected to be, nonpolar, i.e., it has zero charge on every atom. So, if one adds one electron to form theanion, it is added to the NBMO and the results is that the negative charge is determined by the distribution of this electron in the NBMO. Similiarly, if one electron is removed from the radical to generate the carbocation, it is removed from the NBMO, so the positive charge is generated at the positions determined by the electron density in the NBMO.
As an exercise, consult Streitweiser and Coulson’s Dictionary of Pi Electron Calculations in the Chemistry Library to find the coefficients of the pentadienyl system. Use these to calculate the spin distribution in the pentadienyl radical and the charge distributions in the cation and anion. Compare these with the results predicted from resonance theory (i.e., write the three resonance structures for the radical, carbocation and anion).
H. MO’s of Cyclic, Conjugated Systems.
1. The Cyclopropenyl System. Cyclic conjugated systems such as cyclopropenyl, cyclobutadiene, benzene, etc. have higher degrees of symmetry than acyclic systems. For example, benzene has a six-fold symmetry axis, whereas acyclic systems generally have no more than a two-fold symmetry axis. One result of this is that cyclic conjugated systems typically have a number of degenerate orbitals, i.e., MO’s of the same energy but having different spatial distributions of the electrons. The simplest cyclic conjugated system, cyclopropenyl, has one pair of degenerate MO’s which are antibonding. The display of MO’s is shown below. Note that there is one very highly bonding MO, along with the degenerate pair. In contrast to allyl, there is no NBMO. With only a single BMO, this conjugated system is best adapted to accommodate two electrons, i.e., as a carbocation. In the cyclopropenyl radical (three electrons) , one electron is forced to occupy a high energy ABMO, and in the anion (four electrons), two electrons must be filled into the ABMO’s. Recall that in the acyclic, allyl case, the cation, radical, and anion are all relatively stable, since the electrons entering the NBMO do not diminish the stabilization. The total pi electron energy of the cyclopropenyl cation is seen to be much lower than that of the allyl cation. However, the corresponding four electron anion has less stabilization than the allyl anion, and no more than a simple double bond of ethene.
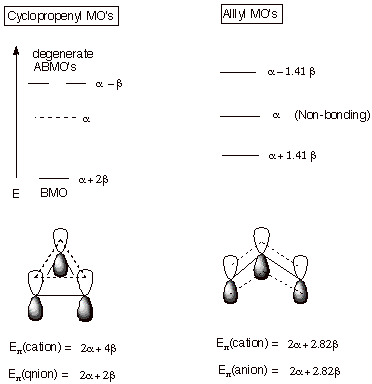
2. The Circle Mnemonic. A good way to recall the distribution of MO’s in simple monocyclic conjugated systems is to use the circle mnemonic. According to this mnemonic device, a polygon of the appropriate number of sides is inscribed inside a circle with one vertex down, corresponding to the unique, highly bonding MO. The MO’s then are arrayed at the vertices of the polygon. Examples for the cyclopropenyl and cyclopentadienyl systems are illustrated. Note that the cyclopentadienyl system now has three BMO’s, so it can accommodate six electrons (that would make this 5 carbon system an anion).
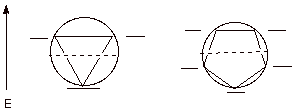
3. Cyclobutadiene. The molecule 1,3-cyclobutadiene has two equivalent resonance structures analogous to the Kekule structures of benzene. We will see, however, that cyclobutadiene could not be more different from benzene in its resonance stabilization and its stability. Following the circle mnemonic, you should be able to easily see that cyclobutadiene has only one BMO, like cyclopropenyl, and that the degenerate pair of MO’s is actually nonbonding. To be neutral, cylcobutadiene must have four pi electrons, but two of them would have to go into the nonbonding level and would add nothing to the pi bonding. Consequently, the pi electronic energy of this molecule is the same as two ethylene units, i.e., it has no resonance stabilization at all. This molecule is highly unstable, even at temperatures not far from absolute zero ( e.g., 20 K). From this we can see that the resonance treatment of cyclobutadiene (two equivalent resonance structures) failes to agree with the MO treatment or with experiment. The problem is that in cyclic systems, the electron count is of crucial importance, and the simple resonance treatement does not take this into account.
4. The Huckel Rule. As you may recall, the
cyclic systems which have special stability can be predicted by the 4n+2 Rule,
where n = 0,1,2,3---. Therefore, monocyclic conjugated systems having
2,6,10,14,---electrons are highly stabilized, but those having 4n electrons
(4,8,12,---) are surprisingly lacking in stability, even in comparison to their
acyclic counterparts. The basis for the Huckel Rule is essentially that the
bonding MO’s of cyclic systems occur in a regular pattern. There is one
highly bonding MO, followed by varying numbers of pairs of BMO’s (zero
for cyclopropenyl, cyclobutadiene; two for cyclopentadiene, benzene, and
cycloheptatriene, etc.). There will always be 1,3,5,7—BMO’s, which
can accommodate 2,6,10,14 – electrons when completely filled.
5. Exercises.
a. Benzene. Construct a display of the MO’s of benzene and fill in the appropriate nujmber of electrons, using one canonical structure as a basis for computing the number of pi electrons. Using the information that the MO’s have energies a+2b,a+b,a+b,a-b,a-b, and a-2b , calculate the pi electron energy of benzene and compare it with the energy of three molecules of ethene. Estimate the resonance energy (delocalization energy) of benzene as the difference in pi electron energy of benzen and three ethene molecules.
b. 1,3-Cyclopentadiene and 1,3,5-cycloheptatriene. Construct energy level diagrams for these systems and fill in the appropriate numbers of electrons for the cation and anion of each. Which species are especially stable? Do these agree with the Huckel Rule.
6. Aromaticity./Antiaromaticity. The circumstance that the delocalization energy of cyclic conjugated systems crucially depends on the electron count, even more than on the number of carbon atoms over which the electrons are potentially delocalized gives rise to the Huckel Rule. The stability of such systems varies from the benzene standard (highly stabilized) to no stabilization whatsoever (or even destabilization). Systems which are unusually highly resonance stabilized, like benzene, the cyclopropenyl cation, the cyclopentadienyl anion, and the cycloheptatrienyl cation are often termed aromatic. Those which are regarded as especially unstable are often referred to as antiaromatic. The concept of aromaticity/antiaromaticity has come to have use in in the concept of transitions states and reaction rates. Not that a transition state is so stabilized as to be an energy minimum, of course, but that a TS which has a 4n+2 cyclic conjugated system is likely to be much lower in energy than a comparable one which has a 4n cyclic conjugated system. Let’s consider some examples of how the concept of aromaticity/antiaromaticity can be used in rationalizing relative reaction rates.
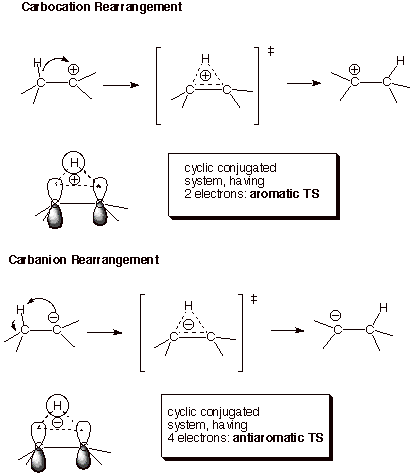
a. Carbocation vs Carbanion or Radical Rearrangements. It is well known that carbocations tend to rearrange very quickly to a more stable carbocation if one is available throught the 1,2 shift of a hydrogen, alkyl, or other group to the previous carbocation center. On the other hand, neither radicals nor anions rearrange to a more stable radical or anion by an analogous process. In fact, carbocations rearrange relatively rapidly even to an equally stable (not more stable carbocation). There is an obvious intrinsic preference for cationic over radical or anionic rearrangements, even when the thermodynamic driving force is factored out. What is the basis for such a strong preference for cationic rearrangement? One way of understanding this preference is based upon TS aromaticity/antiaromaticity. The rearrangement, in either case must take place via a TS which has the hydrogen (or other atom) bridging the migration origin and the migration terminus (the carbon from which it migrates and the carbocation or anion center to which it migrates). This creates a cyclic system of overlap in the TS, so that the electron count is critical. In the carbocation case, there are just two electrons (examine the reactant canonical structure; the two electrons are from the C-H bond which is being broken; the carbocation center, of course, has zero electrons). This TS is therefore aromatic. In contrast, the TS for the anionic rearrangement has four electrons 9the carbanion center contributes two, plus thus of the C-H bond), is is antiaromatic. The difference in the facilities of these two types of reaction is dramatic. Indicentally, the TS for a radical rearrangement has three electrons and is considered nonaromatic. These rearrangements are not observed to occur.
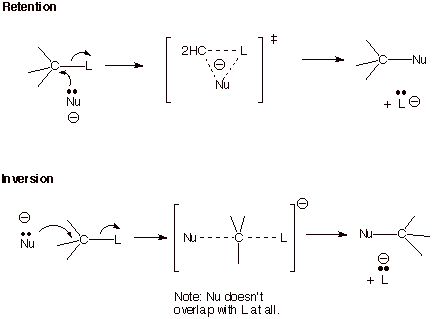
b. The Strong Preference for Inversion over Retention in SN2 Reactions. From the diagram below, we can also see that an SN2 reaction which occurs with retention of configuration at the carbon undergoing substitution (“frontside attack” of the nucleophile, Nu) has a cyclic conjugated system. If we count electrons, we can see that there are four electrons (two from the nucleophile and two from the C-L bond) in the cyclic TS, which is therefore antiaromatic and disfavored in comparison to the invertive process (“backside attack”, because the latter process is nonaromatic (it doesn’t have a cyclic conjugated system).
c. Cycloaddition. It is well known that the Diels-Alder reaction is one of the most facile and useful reactions in organic chemistry. It is no coincidence that the TS for this reaction has a cyclic system containing 6 electrons, i.e., an aromatic TS. In contrast, cycloadditions having 4 or 8 electrons are much more difficult to bring about.
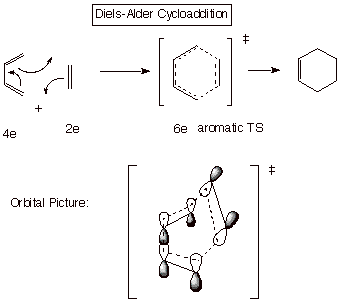
In sharp contrast, the cycloaddition between two alkene units, to give a cyclobutane is very difficult to achieve and typically does not occur in the concerted manner of the Diels-Alder reaction (one bond is formed at a time and high temperatures are usually required). A concerted mechanism would have a TS with antiaromatic character. As an exercise, see if you can draw a dotted line TS structure, such as that shown for the Diels-Alder reaction, for such a reaction.
The Diels-Alder reaction is said to be a [4+2} cycloaddition, because the diene component contributes 4 electrons and the dienophile 2 electrons to the cyclic conjugated system present in the TS. The reaction between two alkenes is termed a [2+2] cycloaddition.
Carbene Additions to
Alkenes. The addition of carbene (CH2) and substituted
carbenes (like dichlorocarbene) to alkenes is an effective way of making
cyclopropane and its derivatives. Singlet carbenes have an electron pair in a
hybrid orbital and also a vacant 2p AO. In adding to the pi bond of ethene or
any alkene, the mode of carbene addition to form two new C-C bonds is rather
unique, but is correctly predicted by the concept of TS aromaticity. Note that
an aromatic TS is set up when the carbene presents its vacant 2p AO to the face
of the pi bond, giving a two electron cyclic TS. If the carbene presents the
filled hybrid AO to the face of the pi bond, an antiaromatic, four electron
cyclic TS is set up. The former is
therefore strongly preferred over the latter. The especially interesting part
is that by presenting the hybrid AO to the face of the pi bond, the methylene
group would be oriented precisely the way it needs to be to form the product
cyclopropane. However, when the vacant 2p AO is presented, the methylene
hydrogens are far out of position in respect to where they will need to be in
the product. What is the solution to this dilemma? Simply that the required
rotation of the methylene group into the proper position takes place after the
transition state, so that any undesirable antiaromatic effects are not felt in
the TS and are overcome by the bonding which is very strong at that point. So the
interesting thing about carbene additions is that the do not take place by the
“Path of Least Motion.”
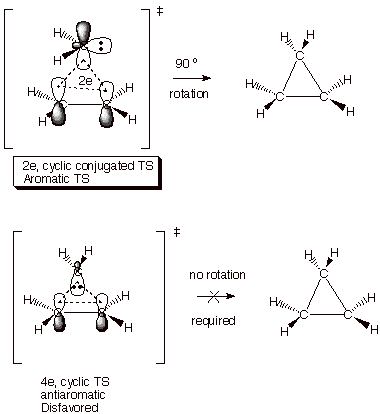
I. Frontier Orbital (FO) Theory and Perturbation
Theory.
1. Filled/Vacant Interactions and HOMO/LUMO Interactions. We already understand that when two AO’s interact, as in the prototype case of the hydrogen molecule, two MO’s are formed, and if two paired electrons are available to enter the BMO, strong bonding results. The requirement for two electrons can be satisfied by having two electrons in one AO (this would be a hydride anion) and zero in the other (a proton), or by having two hydrogen atoms, each with one electron, with the electrons having opposite spin. An analogous situation arises when any two orbitals interact, even if the orbitals are MO’s, although the bonding which results may be much weaker than in the case of molecule formation from atoms. We have already seen that essentially all molecules (simple or complex) have filled bonding BMO’s and vacant antibonding MO’s. If two molecules come into contact, there is therefore always a possibility of bonding interactions between the two, since each molecule has filled MO’s and vacant MO’s. These interactions would result in “supermolecule” formation or complexation, in which the new MO’s are delocalized over the entire complex. In the case of interactions between molecules, the bonding interactions are sometimes modest and may be negated by repulsive nonbonding interactions between the molecules and the unfavorable entropy effect of combining two molecules into one complex molecule. In other cases, relatively stable complexes (e.g., pi complexes) may be formed.
q Any interaction between a filled and a vacant MO can potentially result in a stabilizing interaction because the filled orbital will be stabilized and the vacant one destabilized and two electrons are present, so that only the stabilized MO is occupied. These interactions will be referred to as filled/vacant interactions or F/V interactions.
q Note that interactions between two filled MO’s can also result in the formation of delocalized MO’s, but there will be no net stabilization because both the stabilized and destabilized MO’s are doubly occupied (the four electron situation).
q Similarly, the interactions between two vacant MO’s obviously results in no stabilization, although delocalized virtual MO’s may result.
q Importantly, although any of the potentially many F/V interactions may be stabilizing, the HOMO/LUMO interactions are virtually always the most important ones, because the interaction energy depends upon the energy difference between the F and V MO’s. In the case of the HOMO/LUMO interaction, this energy difference is obviously the smallest. The energy difference between a given HOMO and the corresponding LUMO of the other molecule is sometimes termed the “HOMO-LUMO gap”.
q The approximation in which only the HOMO/LUMO interaction is considered (among all of the possible F/V interactions) is called the Frontier Orbital (or FO) approximation.
2. Symmetry Effects.
In the case of interactions between molecules in which a cyclic conjugated system of orbitals is set up, there is an additional limitation placed upon the interacting F/V MO’s, viz., symmetry. Specifically, the interacting MO’s must have the same symmetry classification. Typically, the MO’s are classified as either symmetric (S) or antisymmetric (A) with respect to a symmetry element, e.g. a plane of symmetry which each interacting molecule has and which is also common to the complex formed between them.
q Antisymmetric means that when the symmetry operation is applied, the electron distribution is unchanged, but the coefficients of all of the orbitals change sign.
q Symmetric means that when the symmetry operation is applied, the electron distribution is unchanged and the coefficients of all of the orbitals remain as they were before the symmetry operation.
q The symmetry element can be almost any element, but most commonly is a plane of symmetry or an axis of symmetry.
q Note that the BMO of the pi bond of ethene is S with respect to a plane of symmetry (coefficients have the same magnitude and sign), but the ABMO is A, because the coefficients of the two carbons have the same absolute magnitude (meaning that when the wave function is squared to obtain the electron density, the electron density is the same at both carbons), but the coefficients of the two carbon atoms are opposite in sign. In the same way, by referring to the MO’s of the allyl system, you can see that the first MO is S, the second A, and the third S. In the butadiene system, the same pattern is followed, the lowest energy MO being S, the next A, the third S, and the highest energy MO A.
J
Applications of FO Theory.
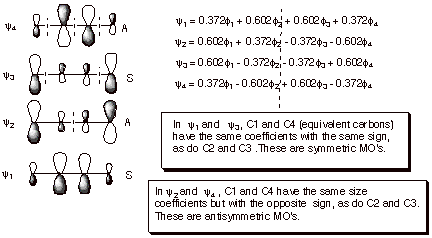
1. Diels-Alder TS. The TS for the Diels-Alder cycloaddition reaction can be considered as approximating a cyclic complex between butadiene (in the s-cis-conformation) and ethene. There are two non-equivalent HOMO/LUMO interactions as shown in the illustration below. In both cases, there is a symmetry match, so that the interactions occur and result in stabilization.
q The diene HOMO is the second MO, which is antisymmetric. The ethene LUMO is the ABMO, which is also antisymmetric. The interactions at both termini of the diene are bonding, so there is net bonding interaction.
q The diene LUMO is the third MO, which is symmetric. The ethene HOMO is the BMO, which is also symmetric. Again, the symmetry match provides that the interactions at both diene termini can simultaneously be bonding.
q Both HOMO/LUMO interactions contribute to the stabilization of this TS.
q The Diels-Alder reaction is an example of a [4+2] cycloaddition, in that the diene supplies 4 pi electrons to the cyclic TS, and ethene supplies 2.
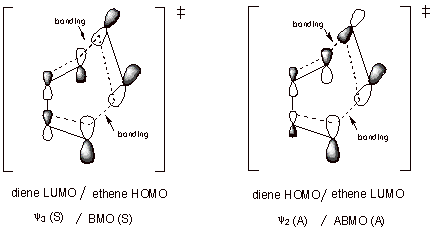
2. [2 + 2] Cycloadditions. You canerify that in the attempted combination of two ethene molecules to give cyclobutane both HOMO?LUMO interactions are not symmetry matched. Although bonding can take place at one terminus, the interaction at the other terminus is antibonding, resulting in no net interaction.
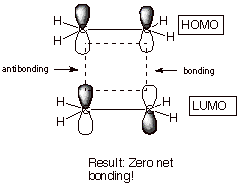

3. Inversion in an SN2
Reaction
In the displacement of a leaving group by an entering
nucleophile, the nucleophile N-) obviously supplies the electron pair (the filled orbital).
To obtain a stabilizing interaction, this orbital must overlap with a vacant
orbital, not the filled bonding orbital of the C-L bond. The vacant orbital
must then be the ABMO corresponding to the C-L sigma bond. Since the latter MO
has a nodal plane between C and L, it is clear that in a purely hypothetical
frrontside (retentive) displacement, the nucleophile cannot interact bondingly
with carbon without simultaneously interacting antibondingly with L. However,
it can interact bondingly with carbon from the backside, without significantly
interacting with L.
There
is another interesting part to this discussion. You might think that overlap
with the small lobe of the sp3 orbital of carbon might be rather
inefficient. However, as we had noted earlier in the case of the
tetracyanoethene anion radical, the ABMO is attenuated in the bonding region
and enlarged in the back lobe, so that overlap is actually enhanced from the
back side when the sigma bond LUMO is involved.
CH 368 Terms and Concepts
Alternant Hydrocarbon
System: A planar,conjugated pi
electron system which contains no odd-membered rings. They may have even membered rings or be purely acyclic or may
contain a mixture of rings and acyclic features (e.g., styrene, i.e.,
vinylbenzene or the benzyl system).
Even
Alternant Hydrocarbon System: An
alternant system which has an even number of carbon atoms in the conjugated
system
Odd
Alternant System: An alternant system
which has an odd number of carbons.
Non-Alternant System: Contains an odd-membered ring in the conjugated
system.
PROPERTIES OF THE MO’S OF ALTERNANT AND NON-ALTERNANT SYSTEMS
q
All alternant hydrocarbon
systems (but not non-alternant systems) have pi MO’s whose energies and
coefficients are paired in terms of energy and coefficients:
o
Paired means
that for every BMO there exists an ABMO the energy of which is exactly as
antibonding as the BMO is bonding. Also, the coefficients at each atom in the
ABMO has the same absolute magnitude as the coefficient at that atom in the
BMO, so that the electron distributions are the same.
q
Odd alternant systems
have, in addition to the paired BMO’s and ABMO’s, a single NBMO.
CYCLIC CONJUGATED SYSTEMS:
Conjugated systems in which all of
the carbons bearing 2pz AO’s form a ring. Monocyclic means
they are contained in a single ring.
PROPERTIES OF THE MO’S OF MONOCYCLIC CONJUGATED SYSTEMS
q
Assuming that the ring has
the shape of a regular polygon, the symmetry of the system gives rise to many
orbital degeneracies (all MO’s except the lowest BMO, and in the case of
an alternant system, its paired ABMO)..
q
The lowest energy BMO is
non-degenerate and highly bonding.
q
Succeeding higher energy
MO’s occur as doubly degenerate pairs.
q
Alternant cyclic systems
have paired MO’s.
q
In non-alternant cyclic
systems, there is of course no orbital pairing.
Resonance Energies. The
lowering in energy of a conjugated system caused by the delocalization of the
electrons over more than two atoms. This is the energy difference between the
real, delocalized system and the energy it would be expected to have if were
not delocalized (i.e., if it were fully localized). Typically, this is given
as:
Ep
-Epo = Energy of the actual pi system – Energy of
the Hypothetical Localized Model
q
The energy of the
actual, delocalized pi system is given as:
Ep = S Nj Ej
q
Where the summation is
over all occupied orbitals (j). Nj is the orbital occupancy (number
of electrons in that orbital) and Ej is the energy of that MO.
q
The BMO of ethene has
energy of a + b, and this MO has an occupancy
of 2, so that the total energy of the pi bond of ethene is 2(a + b) or 2a + 2b.
q
The energy of an
electron localized in a 2p AO on carbon is the coulomb integral, a. Since a carbanion has 2 electrons localized on a single carbon, it has
an electronic energy of 2a.
A carbocation site has no electrons in
the 2p AO, so it contributes nothing to the pi electron energy.
q
The energy of the
localized model (Epo)
calculated as the sum of the energies
of all the ethylenic pi bonds, carbocation centers, radical centers, and/or
carbanion centers.
Charge Distributions
The charge density (Qi) at a specific atom
(i) is calculated as the difference between the electron density at that atom
and the electron density that the atom should have when neutral (uncharged). In
the pi electron approximation, that would be one, since a neutral carbon (as in
the methyl radical) would have a singly occupied 2pz AO.
Qi = 1- S Njaij2 = 1- qi
Where the summation is over
all the occupied MO’s (j). and aij is the coefficient of atom
i in a given MO (j).
q
Alternant hydrocarbon
systems (if they are not ions or ion radicals, i.e., they are overall
electrically neutral) have zero charge densities at all carbons. Note that this
is not true if there is a heteroatom in the sytem (atom other than carbon, such
as oxygen or nitrogen). Thus, an etheneic double double is nonpolar, but a
carbonyl double bond is highly polar.
q
Non-alternant hydrocarbon
systems can have non-zero charge densities at the various atoms.
Frontier Orbitals
The highest energy occupied MO is designated the HOMO,
and the lowest energy vacant (unoccupied) MO is termed the LUMO. In a radical
species, the orbital which contains the odd electron is termed the SOMO (singly
occupied MO).
Spin Densities or Odd Electron Densities
Since the SOMO of a radical (or ion radical) species
exclusively controls the distribution of the spin (the odd electron), the spin
density or odd electron density (or radical character) can be calculated just
from the SOMO coefficients. Square the coefficient of a given atom in the SOMO
to obtain the spin density (ri).
ri = ci,SOMO2
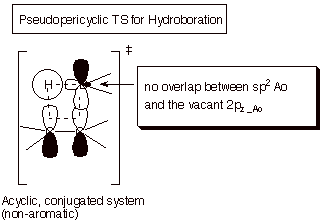
An addendum concerning mechanisms
You may recall that we have written the mechanism of the HCl addition to alkenes as a stepwise one, in which one bond is made first (the new C-H bond), followed by a second step in which the C-Cl bond is made. The question might well be asked, why doesn’t this reaction occur in the concerted manner of the hydroboration reaction? The answer is that the concerted addition of HCl would necessarily have a cyclic, conjugated transition state with four electrons, which would be antiaromatic. But then, why is the hydroboration reaction permitted to be concerted. The answer to this is especially interesting and informative. In the case of hydroboration, the Ts is not really cyclic (or pericyclic), because the sp2 AO on boron (which is a part of the B-H bond) is orthogonal to (does not overlap at all with) the 2pz AO on boron, which actually is the orbital which initiates bonding with the ethenic pi system. Such a reaction is called “pseudopericyclic” because it appears to have a cyclic system of orbitals, but this system is not completed. It is, in effect, an acyclic orbital system. Acyclic, conjugated systems are not sensitive to electron count.