Unit 8: Electron Transfer Reactions
Most of the reactions by which anion radicals and cation radicals are formed are electron transfer (ET) reactions. Since electron transfer is the simplest of all chemical reactions, its study is especially fundamental, and we might expect to find some extremely fast reactions in this domain. First, let’s look at three different methods of effecting electron transfer in the organic laboratory.
Chemical Methods. As an example of the formation of anion radicals by chemical methods, we have already considered the transfer of an electron from sodium or potassium metal to a neutral substrate, such as benzophenone (to form the ketyl). A chemical method for forming cation radicals which was emphasized was the use of a stable aminium salt to ionize an electron from an organic substrate.
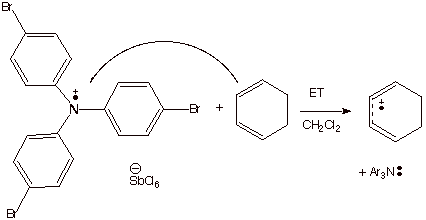
Electrochemical Methods. We can also form anion and cation radicals by electrochemical reduction or oxidation at an appropriate electrode at an appropriate reduction or oxidation potential (Scheme 2).

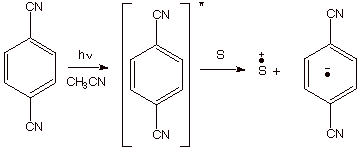
Photochemical Methods. Besides the chemical and electrochemical methods of generation, there are also some very efficient photochemical methods. The most common approach is to use a photosensitizer to absorb uv light, and to allow the resulting excited state of the photosensitizer to remove an electron to an organic substrate (or to transfer an electron to the organic substrate). This is illustrated for the case of 1,4-dicyanobenzene as the photosensitizer. If, for example, 1,3-cyclohexadiene is the substrate, this results in a cation radical chain process which forms the previously mentioned Diels-Alder dimers.
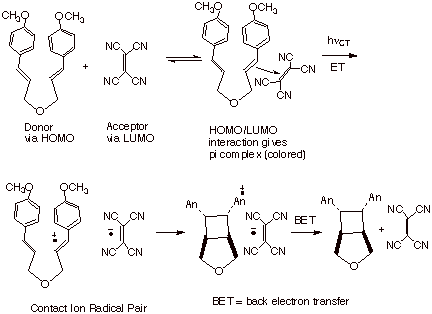
Ion Radical Pairs
Solvent-separated Ion Pairs. The transfer of an electron from a neutral substrate to an excited state, neutral molecule (more specifically to the LUMO of this molecule) is seen to result in the formation of an ion pair; more specifically a cation radical/anion radical pair. A fundamentally important question is what kind of ion pair is this, i.e., is it a contact ion pair in which no solvent molecules separate the two oppositely charged ions, or is it a solvent-separated (or solvent-penetrated) ion pair. If the former, electron transfer must have occurred when the two species were in direct contact. If the latter, electron transfer must be occurring through the solvent. We shall see that the latter is actually the case in the photosensitized electron transfer (PET) ionization of organic substrates as described above.
Tight or Intimate Ion Pairs. An alternative method has been devised to specifically generate the contact (or tight or intimate) ion radical pair. However, this approach is only applicable to cases in which the single electron donor and the single electron acceptor for a pi complex. Since pi complexes (donor/acceptor complexes) typically have a long wave length absorption corresponding to the completion of the ET by excitation, this ET process can be readily brought about by irradiation of the pi complex at the long wave length at which only the pi complex absorbs. An example of this is shown below, in which tetracyanoethylene acts as the acceptor and an ionizable organic substrate acts as the donor. The formation of the cation radical then is signaled by the rapid, intramolecular cyclobutanation of the substrate.