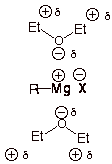Formation of Grignard Reagents from Organic Halides
q
The carbon atom of
organic halide which is directly attached to the halogen is, of course,
electrophilic. This electrophilic reactivity can be switched to nucleophilic
reactivity by conversion to an organomagnesium halide, i.e., a Grignard
reagent.
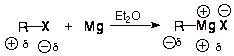
q
Especially note that the
carbon-magnesium bond in a Grignard reagent is polar covalent with carbon being
the negative end of the dipole. Thus the nucleophilicity of carbon in a
Grignard reagent. Note also that the magnesium-halogen bond is largely ionic,
as shown in the structure above.
q
We will consider the
synthetic applications of the nucleophilic carbon atom present in Grignard
reagents and other organometallic reagents in a later chapter.
q
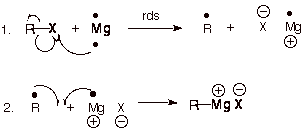
The mechanism of
formation of a Grignard reagent is shown below. Like the other reactions
considered in this chapter, it also involves radical intermediates. There is
one major difference, however. Grignard formation does not involve a radical chain mechanism. It is a non-chain radical reaction.
q
Note that the first step
is rate-determining and involves the transfer of one electron from Mg (which
has two electrons in its valence shell) to the carbon-halogen bond. This forms
Mg+1, which is a radical. This then couples with the alkyl radical
formed.
q
Diethyl ether is an
especially good solvent for the formation of Grignard reagents because ethers
are non-acidic (aprotic). Water or alcohols would protonate and thus destroy
the Grignard reagent, because the Grignard carbon is highly nucleophilic. This
would form a hydrocarbon. But Grignard reagents are stable in ethers.
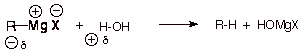
q
Another reason that
ethers are good solvents for Grignard reagents is that the MgX bond is ionic
and thus benefits greatly from being effectively solvated. The formation of
ions in very nonpolar solvents, where they would not be effectively solvated is
very difficult. Ethers are surprisingly good at solvating cations, because the
C-O bond is relatively polar, thus allowing the oxygen end of the ether dipole
to solvate and stabilize (electrostatically) the magnesium ion.