ACID BASE
EQUILIBRIA INVOLVING CARBONYL COMPOUNDS
![]()
q
The qualitative
criterion for whether an acid/base reaction goes to completion or not is: The
stronger acid is converted to the weaker acid. Since the pKa of the
ketone is ca. 19 and that of water is ca. 16, water is the stronger acid.
Therefore the equilibrium lies to the left.
q
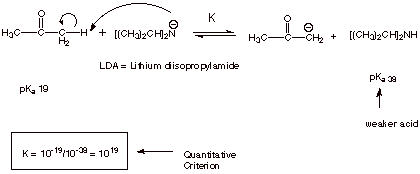
The quantitative
criterion for obtaining the equilibrium constant K for this acid/base reaction
is:
K = K(Reactatnt Acid)/K(Product Acid).
In the present application the K for the reactant acid, the ketone, is 10-19,
while the K for the product acid, water, is 10-16. The ratio is
therefore 10-19/10-16 or 10-3.
QUANTITATIVE CONVERSION TO THE ENOLATE
q
A much stronger base
than hydroxide ion is needed to do this.
q
Quantitative conversion
is important because if all of the base used (like hydroxide ion) is not
consumed in forming the enolate, it will be available to compete with the
enolate: both are good nucleophiles.
q
Although the simple
amide (NH2_ anion) is strong enough, it is also a pretty good nucleophile
and adds readily to the carbonyl carbon. This process is irreversible because
the result anion is an alkoxide anion, whereas the reactant anion was an amide
type anion (much less stable). Consequently, the hindered amide base LDA is
used because it is equally strong as a base and steric hindrance slows down the
addition to the carbonyl carbon in comparison with abstracting the small
proton.