TOP
TABLE OF CONTENTS FOR THIS
CHAPTER
BRONSTED ACIDS AND BASES IN
ORGANIC CHEMISTRY
- You should know that Bronsted-Lowry acids are proton
donors, and Bronsted bases are proton acceptors.
- You should be able to write simple Bronsted acid/base equations
using curved arrows to show electron flow. The curved arrow should start at
an electron pair, either an unshared pair or an electron pair which is involved
in a covalent bond.The curved arrow should point to the nucleus (in this case
always a proton) to which the electron pair will bind in the product(the right
hand side of the equation).
- You should also know that the conjugate base of a Bronsted
acid is the species formed when a proton is lost from that acid (e.g.,
hydroxide ion is the conjugate base of water; water is the conjugate base
of the hydronium ion). Similarly, the conjugate acid of a Bronsted base
is the species formed when that base is protonated.Thus, water is the
conjugate acid of hydroxide ion, and hydronium ion is the conjugate acid of
water. In other words, water and hydroxide ion are a conjugate acid/conjugate
base pair.
Quantitative Measurement of
Acid and Base Strength
- You should know that the dissociation of a Bronsted-Lowry
acid in water produces the conjugate base of the Bronsted acid, along with
the hydronium ion. The equilibrium constant K for this dissociation measures
the extent of hydronium ion formation and thus, effectively , how strong the
Bronsted acid is. In this case strength refers to the relative tendency of
the acid to protonate water.
- The acid dissociation constant of the Bronsted-Lowry acid
(Ka) is the quantity which is actually used to measure the strength
of the acid quantitatively (rather than the equilbrium constant itself). This
is simply the product of the hydronium ion concentration and the concentration
of the conjugate base of the acid (both on the product side of the equilibrium),
divided by the concentration of the Bronsted acid itself(on the left hand
side of the equation). Notice that the concentration of water, which is also
on the left hand side of the equation, is omitted from the expression, since
this is effectively constant in dilute aqueous solution. Thus the acid
dissociation constant is the equilibrium constant K multiplied by the constant
concentration of water in water.
- Frequently, the pKa, which is the negative
common log of the acid dissociation constant is used as a more conveniently-sized
number to measure the acidity. Since this is the negative log,
a large positive pKa means a large negative exponent of the acid
dissociation constant, Ka. this corresponds to a weak acid (Ka
greater than 1 indicates more product than reactant; Ka less than
1 indicates more reactant than product; the hydronium ion is on the product
side). Conversely, a negative value of the pKa corresponds to a
strong acid.
The Effect of Structure upon
Acidity
Acid/base reactions are of tremendous importance in organic
chemistry, as they are also in inorganic and biochemistry.Further,the acidity
of hydrogen-containing compounds varies remarkably from one compound to another.In
order to understand why acidities of Bronsted acids vary so widely, and to be
able to systematically understand the trends in acidity,we will consider five
main effects of structure upon acidity: (1)Periodicity within a column of the
periodic table(equivalent to Bond Strength Effects); (2)Hybridization (3)Resonance
Effects (4)Inductive Effects and (5)Electronegativity effects.
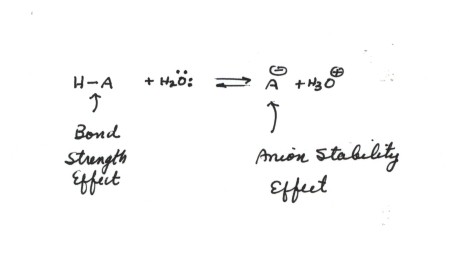
Consider the following acid
dissociation:
The factors which would favor dissociation, i.e., enhance acidity,
are:
- A weaker H-A bond, because this destabilizes the
left hand(reactant) side of the equation. This is the bond strength affect.
A stronger H-A bond tends to decrease the acidity because the bond is harder
to break. A weaker H-A bond increases acidity.
- A more stable anion, because this stabilizes the
right hand side of the equation. This is the Anion Stability Effect.
It is an important
general rule of thumb that Anion Stability usually exerts
the strongest effect upon acidity, i.e., is dominant over bond strength effects.
The one special type of exception will be considered shortly.
Anion stability, in turn is affected by (1)resonance
stabilization,(2) inductive effects, (3) hybridization effects and (4)electronegativity
effects.
We discuss first the exceptional, but important case where bond strength
effects predominate.However, even when they are not predominant, it should
always be remembered that they always are operative and tend to increase or
decrease acidity according to whether the bond is weaker or stronger. If there
are opposing anion stability effects, the net effect will usually be qualitatively
that predicted by considering the anion stability effect.
A Case Where Bond Strength Effects
Predominate Over Anion Stability Effects
***The Relative Acidities of
H-X, i.e., of the hydrogen halides.****
- The pKa's of H-F,H-Cl,H-Br, and H-I are respectively
3.5,-7,-8,and -9. That is, H-F is the weakest acid and H-I the strongest
(recall that a negative pKa corresponds to a strong acid.
- Based upon anion stability, the opposite trend should
be observed, since fluoride ion should be the most stable of the halide
anions (refer to the electronegativity of fluorine, which is the greatest
of the halogens.)
- However, the H-F bond is extremely strong and hard to
break, so the bond strength effect is acid weakening and is predominant
here. The bond dissociation energies of the H-X bonds are, respectively,
136 (HF),103 (HCl),88 (HBr), and 71 (HI). So, the bond strength differences,
being large, are greater than the differences in anion stability, causing
the former effect to predominate. The effect is largest for fluorine, so
that there is relatively little difference in the acidity of the other halogen
acids. Thus the two effects are almost, but not quite in balance for these
latter three H-X's.
- Similar considerations apply for comparisons in any case
involving atoms in the same Group of the Periodic Table. But, rarely are
bond strength effects dominant over anion stability effects in any other
comparative situation.
ELECTRONEGATIVITY EFFECTS
Anion stability is strongly
affected by the electronegativity of the atom upon which the negative charge
rests, i.e., the ability of the atom to stabilize additional electrons.
- For example, negative charge is more stabilized by a
fluorine nucleus than a nitrogen nucleus because fluorine has a nucleus
which has a positive charge of +9 (nine protons), whereas that of nitrogen
has only +7. So pure electrostatic effects(potential energy of attraction
of the negatively charged electron to the positively charge nucleus) stabilize
electrons on fluorine more than on other atoms. In general, electronegativity
is a fairly good neasure of the ability of an atom to stabilize negative
charge.
- The electronegativity of fluorine is also greater than
that of chlorine , bromine, or iodine, i.e., electronegativity decreases
down a column of the Periodic Table. This is because the electron with chlorine,
for example, must enter an orbital in the third main shell, which is much
further from the nucleus than the second main shell, which would be involved
for fluorine. The potential energy of attraction between the electron and
the nucleus is inversely proportional to the distance. In the case of a
comparison in the same row of the periodic table, the distance factor is
the same, because the electrons are entering the same main shell, so it
is the charge on the nucleus which is the determining factor of the electronegativity
of the atom.
- The pKa's of methane,ammonia,water, and HF
are respectively 51, 38, 15.7, and 4.0, i.e., acidity sharply increases
upon going from left to right in the periodic Table. This follows the order
of anion stability, because the methyl anion is less stable than the amide
anion, than the hydroxide anion, than fluoride anion. That is, negative
charge is most stable on F, the most electronegative atom, and least stable
on carbon, the least electronegative atom of this series. It is true that
bond strength effects are in the opposite direction and would tend to make
HF the weakest acid, but anion estabilization effects are larger. So it
is good to remember that anion stabilization effects are dominant in all
cases except when comparisons are made within one group of the periodic
table.
HYBRIDIZATION EFFECTS
***An especially interesting
case to consider is the comparison between anions in which the negative charge
is on the same atom but the hybridization state of the atom is varied. The most
important case here is that of carbon, which, as you know, has three distincet
hybridization states.***
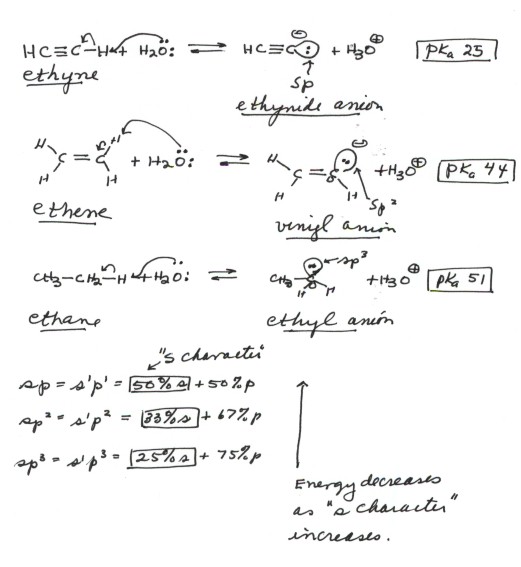
- The acidity of ethyne(acetylene) is much greater than
than of ethene, which is much greater than than of ethane. The relevant
pKa's are 25, 44, and 51.
- The bond strengths would predict the opposite effect,
since the C-H bond of ethyne is stronger than that of ethene than that of
ethane. So, anion stabilization effects must be operative.
- The relevant anions, as shown in the figure, are the
ethynide anion, in which carbon is sp hybridized, the vinyl anion (trigonally
or sp2 hybridized), and the ethyl anion (tetrahedrally or sp3
hybridized).The former is the most stable and the last-named one least stable,
so that the rule of controlling anion stability is obeyed.
- It is very important to understand the reason for
the profound difference in acidity of C-H bonds as a function of their hybridization
state.The key point here is that the lower the energy of the orbital
in which the unshared pair resides in the anion, the more stable will be
the anion. In the ethynide anion, the unshared pair is in a digonal orgabital
which is 50%s and 50%p. In the vinyl anion, the unshared pair is in a trigonal
orbital which is 33%s and 67%p, while in the ethyl anion the unshared pair
is in a tetrahedral orbital which is 25%s and 75%p. Since the energy of
an s orbital is much lower than that of the corresponding p orbital, the
greater the "% s content" or "s character" of the orbital
the lower its energy.Please note that ethyne is almost 20 powers of ten
more acidic than ethene.
- It is also important to note that ethyne is 13 powers
of ten more acidic than ammonia, even though in the latter case the negative
charge in the amide anion is on a nitrogen atom, which has a much higher
electronegativeity than carbon. Thus hybridization effects are impressively
large, and can cause carbon to appear as even more electronegative than
nitrogen, when the former is in the digonal hybridization state.
RESONANCE EFFECTS
WE ARE STILL FOCUSING UPON ANION STABILITY
AS THE DOMINANT FACTOR IN DETERMINING THE RELATIVE ACIDITIES OF BRONSTED-LOWRY
ACIDS. EVEN WHEN THE NEGATIVE CHARGE IS LOCATED ON THE SAME ATOM (SO THAT ELECTRONEGATIVITY
IS NOT A FACTOR), AND WHEN THE HYBRIDIZATION STATE OF THE ATOM ISN'T DIFFERENT(SO
THAT HYBRIDIZATION EFFECTS ARE NOT IMPORTANT), A SERIES OF ANIONS (CONJUGATE
BASES OF THE BRONSTED-LOWRY ACIDS) CAN BE DIFFERENTIALLY STABILIZED BY RESONANCE.
AS YOU SHOULD RECALL, DELOCALIZATION OF THE CHARGE OVER MORE THAN ONE ATOM
RESULTS IN RESONANCE STABILIZATION. AN EXCELLENT EXMAPLE IS AVAILABLE IN
THE COMPARISON OF THE ACIDITIES OF ALCOHOLS(RO-H) WITH CARBOXYLIC ACIDS (RCOOH):
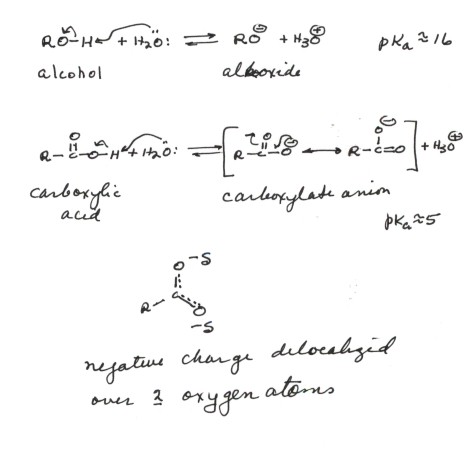
- The conjugate base of an alcohol is an alkoxide anion
(RO-). In the case of ethanol, it is ethoxide anion. In such
an alkoxide ion, the negative charge is essentially localized upon oxygen.
This being an electronegative atom, the negative charge is fairly stable
there, so that alcohols are modestly acidic, comparable to water. This is
reasonable because in the conjugate base of water, the negative charge is
also located upon an oxygen atom.
- The conjugate base of a carboxylic acid is a carboxylate
anion. In the case of ,say, acetic acid, it is acetate anion. In such
a carboxylate anion, the negative charge is also upon oxygen, but because
of resonance effects it is delocalized over two oxygen atoms. Therefore
additional stabilization of the anion results, so that carboxylic cacids
are typically about 11 powers of ten more acidic than alcohols.
INDUCTIVE EFFECTS
Note that this section is quite different form
the one in our text. For the purposes of this course, please neglect the text
discussion and consider the present discussion as replacing it.We are again
considering ways in which the anion produced by deprotonating a Bronsted-Lowry
acid can be stabilized. The final way which we want to consider is by stabilizing
the site of negative charge by interaction with other dipoles which may be present
in the molecule. Typically, this kind of effect can be somewhat complex, but
the overall result of this stabilization (or destabilization) is referred to
as "the inductive effect". As an example, we consider the comparison
of acetic acid and chloroacetic acid.
- The pKa of acetic acid is 4.76, while that
of chloroacetic acid is 2.86, i.e., the latter is almost 100 times more
acidic than the former.
- The conjugate base in each case is a carboxylate anion,
so electronegativity effects and resonance effects should be equal in each
case. What makes the chloroacetate anion relatively more stable than the
acetate ion? This is the inductive effect.
- We recall that a C-Cl bond is substantially polar in the
sense of carbon being partially positively charged and chlorine partially
negatively charged, because of the electronegativity difference betwee these
two atoms. The dipole in this bond is oriented with its positive end closer
to the two sites of negative charge(the oxygens). There is therefore a stabilizing
electrostatic attraction between the positive end of the C-Cl dipole with
the negative charge of the anion. This is larger than the destabilizing(repulsive)
interaction of the negative charge with the chlorine end of the diple, because
the distance between the latter and the negatively charged oxygens is greater.
Thus, there is a net electrostatic attraction and stabilization in the anion
because of the (1)existence of the dipole and (2)the orientation of the
positive end toward the negatively charge oxygens.
- If the dipole were oriented with the negative end toward
the anion site, there would be a destabilization, and a decrease in acidity.

EQUILIBRIUM POSITION IN ACID/BASE
REACTIONS
It is very important to realize that pKa
values refer to the equilibrium in which a Bronsted-Lowry acid transfers its
proton to water to give a hydronium ion. Not only is the solvent water, but
the base which accepts the proton (the Bronsted-Lowry base) is water.When a
Bronsted-Lowry acid reacts with a base other than water, how can we predict
the position of the equilibrium, qualitatively and quantitatively? That is,
how can we know whether the reaction will or will not proceed to completion
and how far to completion? We will consider both a qualitative criterion and
a quantitative one. The former will suffice if we only need to know if the reaction
proceeds primarily to the right or primarily toward the left.
THE QUALITATIVE CRITERION
- It is important to realize that if a B-L acid reacts with
a B-L base, the result is the formation of a new B-L acid and a new B-L
base. Thus, if H-A reacts with B-, the result is the formation
of A- and H-B. Thus two B-L acids (HA and HB) are involved,one
on the reactant side (the left hand side of the equation) and one on the
product side (the right hand side of the equation).
- The simple qualitative criterion is that the stronger
B-L acid is converted to the weaker B-L acid (the same thing can be stated
in tersms of B-L bases.
- Strength of B-L acids, is of course judged by pKa
values. If we know or are provided with a table of such values we can always
tell which is the stronger acid and therefore which will be converted to
the weaker acid.
- If we consider the reaction of acetate ion with ethanol,
the products would be acetic acid and ethoxide ion. On the left, we have
ethanol as the B-L acid, and on the right acetic acid. From its lower pK
value, we know that acetic acid is the stronger acid, so it will be converted
primarily to the weaker acid, which is ethanol. Thus, as written, the reaction
proceeds to the left.That is, as written it does not go to completion.
- As a further example, consider the reaction of acetic
acid with ammonia. The products of proton transfer would be acetate anion
and ammonium ion. The acid on the reactant (left hand) side is acetic acid,
that on the right hand (product) side is ammonium ion. Which is stronger?
The pK of the ammonium ion is 9.24, while that of acetic acid is 4.76. The
lower pK corresponds to the stronger acid. So this reaction does proceed
toward the product side to give the weaker acid, ammonium ion.
THE QUANTITATIVE CRITERION
We can do much better than a simple qualitative
criterion merely by using the pK values which we would need anyway for even
a qualititative criterion.
- The simple rule is that the equilibrium constant for the
given reaction of a Bronsted-Lowry acid with a general B-L base is the quotient
of the Ka values of the acid on the reactant side divided by
that of the acid on the product side. Be careful to remember that it is
R/P, not the other way around. (This is not like writing an equilbrium constant
in terms of concentrations).
- In our first example, the reaction of acetate ion with
ethanol to give acetic acid and ethoxide ion, the equilibrium constant K
for the reaction as written is K(ethanol)/K(acetic acid). In the second
case, the equilibrium constant K for the reaction as written is K(acetic
acid, which is the reactant acid)/K(ammonium ion, the product acid). In
the first case the K is much less than one, as seen from the negative exponent
in the illustration, whereas in the last case, K>>1.
- You should be able to calculate the equilibrium constants
of such reactions given the appropriate K or pK values.

LEWIS ACIDS/BASES
The concept of B-L acidity/basicity focuses
on the proton, which is of course, a very important species in both inorganic
and organic chemistry. More specifically, it focuses upon the transfer of
a proton from one species to another, to form a new covalent bond. Professor
G.N.Lewis broadened the concept of acidity/basicity so that covalent bond
formation processes which do not involve the proton may be included in the
same class. A B-L acid donates a proton (with no electrons) to a base which
contributes both electrons to the resulting covalent bond.
Lewis extended the concept of acidity/basicity by defining a Lewis acid as
any chemical species which can form a covalent bond by accepting two electron
from a base. A Lewis base, by the same token, is
any species which can supply a pair of electrons to form a covalent bond.
Any Bronsted-Lowry base is therefore also a Lewis base and conversely. All
that is required is an available electron pair. In contrast, there are many
Lewis acids which are not Bronsted acids, and indeed which may not contain
an available proton at all.
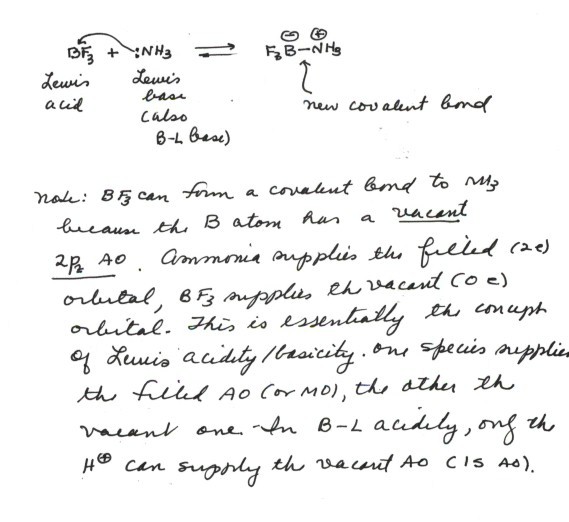
- The reaction between ammonia (NH3) and BF3
is an excellent example. Ammonia is a B-L base and also a Lewis base, because
it can supply an electron pair to form a covalent bond. But boron trifluoride
is by no means a B-L acid, since it has no protons. Nevertheless, it reacts
with ammonia in a fashion similar to a B-L acid, by forming a covalent bond,
using the same electron pair of ammonia that a B-L acid would use. In this
case the new bond is a B-N bond.
BACK TO THE TOP OF THIS PAGE
ON TO THE STEREOCHEMISTRY
CHAPTER
BACK TO THE BAULD HOME PAGE