TOP
Stereochemistry
TABLE OF CONTENTS FOR THIS
CHAPTER
- Isomers:Definitions
- Constitutional Isomers
- Stereoisomers
- Chirality
- Symmetry Elements
- Nomenclature for Enantiomers
- Two Stereogenic Centers
- Two Equivalent Stereogenic Centers
- Comparative Properties of Enantiomers/Diastereoisomers
- Optical Activity
- Racemic Mixtures
- Optical Purity
- Resolution of Enantiomers
- Kinetic Resolution
Isomers:Definitions
You are already familiar with the concept of isomers: different
compounds which have the same molecular formula. In this chapter we learn
to make distinctions between various kinds of isomers, especially the more subtle
kind of isomers which we call stereoisomers.
- Constitutional Isomers: Isomers which
differ in "connectivity". The latter term means that the difference
is in the sequence in which atoms are attached to one another. Examples of
isomers pairs which are consitutional isomers are (1)butane and methylpropane,i.e.,
isobutane, which are different in that butane has a sequence of four carbon
atoms in a row, but isobutane has a three carbon chain with a branch (2)dimethyl
ether and ethanol--the former has a C-O-C chain, while the latter has a C-C-O
chain (3) 1-pentene and cyclopentane--the former has an acylic chain of 5
carbons, while the latter has a 5-membered ring.
- Stereoisomers: Isomers which have
the same connectivity. Thus all isomers are either constitutional or stereoisomers.
Stereoisomerism is a more subtle kind of isomerism in which the isomers differ
only in their spatial arrangement, not in their connectivity. Cis- and Trans-1,4-dimethylcyclohexane
are a good example of a pair of stereoisomers.
Stereoisomers
We have just seen that
there are two major types of isomer, but now it is necessary to further notice
that their are two sub-types of stereoisomers:
- Enantiomers: Stereoisomers which are mirror images
- Diastereoisomers: Stereoisomers which are not mirror
images
The examples of cis- and trans-1,4-dimethylcyclohexane are of
the latter type, that is , they are diastereoisomers. Cis- and trans-isomers in
general are diastereoisomers. They have the same connectivity but are not mirror
images of each other. Enantiomers are mirror image isomers. This is the very most
subtle way in which two chemical compounds can differ:In an overal sense, then
, there are three types of isomers: (1)constitutional isomers (2)diastereoisomers
and (3)enantiomers in order of increasing subtlety of difference. Since we
have previously considered constitutional isomerism, and since the difference
between diastereoisomers and enantiomers rests upon the concept of mirror image
isomerism, we must now consider this latter phenomenon in greater detail.
Mirror Image Isomerism
To be isomers, molecules must not be identical. The test for
"identicality" is one of superimposability. In a sample of
butane, all of the molecules are identical because they can be superimposed upon
one another in some conformation. The same is true of ethanol or propanol or 1-butanol,
but in the case of 2-butanol there are two isomeric forms which can not be superimposed.
They do not differ in connectivity, obviously, or they wouldn't both be called
by the same name (2-butanol). They also don't have a cis or trans prefix, to indicate
that they are diastereoisomers. They have a very specific, unique relationship
to one another, the same relationship which exists between an object and its mirror
image. A key aspect of this difference, as we all know, is that a mirror acts
to interchange left and right hands.
CHIRALITY
- A molecule or object which is not identical to(i.e., non-superimposable
upon) its mirror image molecule or object is said to be chiral. This
means it resembles a human hand in that the left and right hands are not superimposabile
but can be readily distinguished (at least by some of us). By the same token,
a molecule or any object is said to be achiral if it is identical to
(superimposable upon) its mirror image molecule or object. Many molecules
are achiral, but many are chiral, especially complex molecules such as are
found in biological systems.How can we anticipate when a molecule is chiral
and therefore has an isomer (an enantiomer) or when it is achiral and has
no enantiomer?
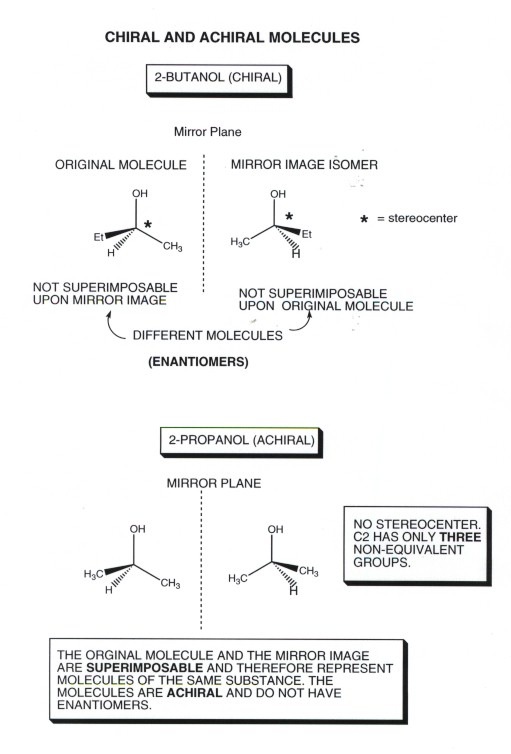
- Consider 2-butanol, which is an example of a chiral
molecule. The illustration below (hopefully) shows that the mirror image
of one 2-butanol isomer is non-superimposable upon the original molecule.
Your can verify this by making models, but you can also visualize trying to
superimpose the two by sliding one structure over (mentally) on top of the
other.We can, for example, slide B over to A and superimpose the OH, the central
C, and its attached H of the B molecule over the corresponding gorups of the
A molecule, but the ethyl group on B sits over the methyl group of A, and
the methyl group on B superimposes upon the ethyl group of A. The two molecules
have all the same kinds of bonds and are extremely similar, but are mirror
image isomers. We will learn how to name the two different enantiomers shortly.
- Although 2-butanol is a chiral molecule and therefore has
two enantiomers, the very similar molecule 2-propanol is achiral and
does not exist as an enantiomeric pair. In the illustration, you can see that
B slides over onto A with all corresponding groups superimposing perfectly.
Many simple molecules are of this kind. How can we predict whether a molecule
is chiral or achiral?
- One of the simple ways is to use the concept of a stereogenic
center. If a molecule has a single stereogenic center it will necessarily
be chiral. The most common kind of stereogenic center is a carbon (or other
atom) which has four different atoms or groups directly attached to it.
You can see that the central carbon of 2-butanol (the one marked by an asterisk)
is a stereogenic center, having H,OH,methyl, and ethyl groups attached. Since
it has just a single stereogenic center , it must be chiral. On the other
hand, 2-propanol has no stereogenic center and is achiral. The corresponding
carbon atom of 2-propanol has an OH,H, and two methyl groups attached. Of
course, no methyl carbon atom or methylene carbon can be chiral since these
groups automatically have at least two identical groups (H's) attached. We
will see a little later what happens when we have more than one stereogenic
center.
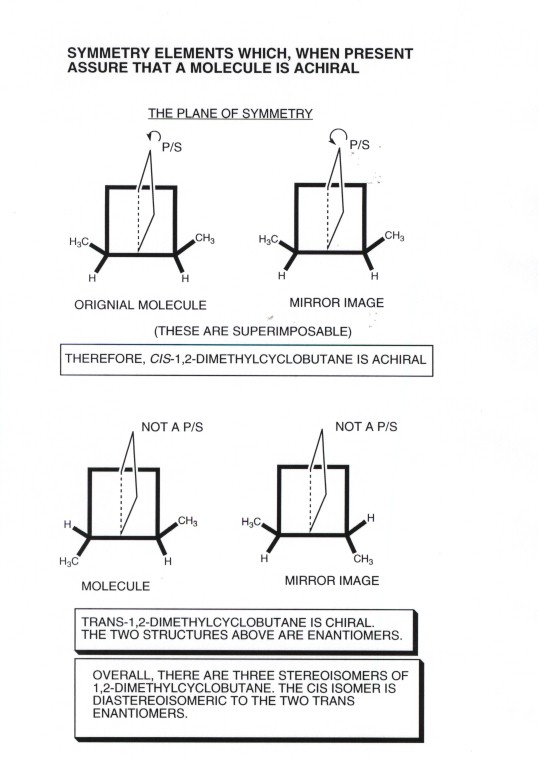
- The second method, especially useful when there is more
than one stereogenic center, is the use of symmetry elements.If the
molecule or object has either a plane of symmetry or a center of symmetry
it is achiral. The examples shown below refer to cis- and trans-1,2-dimethylcyclobutane,
The former of which is achiral and the latter chiral. They both have two stereogenic
centers, viz., the ring carbons which have the methyl and hydrogen groups
attached, but one molecule is chiral and the other achiral. This emphasizes
the point that a molecule or object is guaranteed to be chiral only if it
has a single stereogenic center. If it has more than one stereogenic center,
it may be either chiral or achiral. Note that in the cis isomer, the
two methyls are on the same side of the ring and are equidistant from the
plane of symmtery which runs through the center of the ring perpendicular
to the ring. In the trans isomer, the methyls are on opposite sides
of the ring, so that where there is a methyl group on the right there is a
H on the left.
- What is the relationship between the cis and trans
isomers of 1,2-dimethylcyclobutane??? They are diastereoisomers, having
the same connectivity but obviously not being mirror images of each other.
To sum up, there are three isomers of 2,3-dimethylcyclobutane, a single
cis isomer, and two enantiomeric trans isomers.
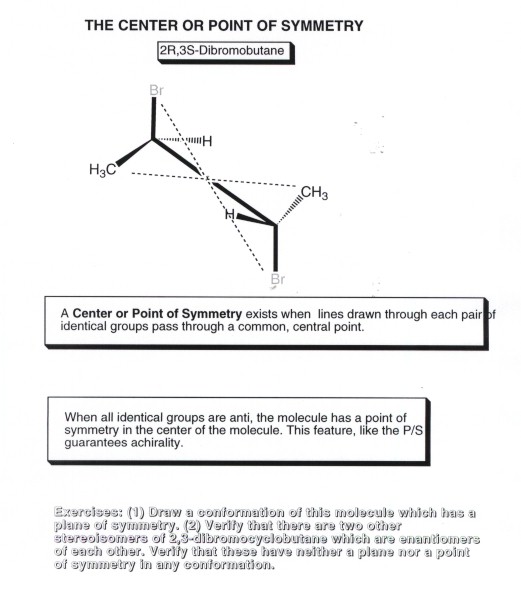
- The plane of symmetry is relatively easy to find
and is the most common one to look for, but one other element of symmetry
also guarantees an achiral molecule, and that is the center of symmetry.
This is a point in the molecule for which any line drawn through the point
will encounter identical components of the object at equal distances from
the center of symmetry.In the case illustrated, 2,3-dimethylbutane (the so-called
meso isomer), the center of symmetry is at the center point of the C2-C3 carbon-carbon
bond. One of the dotted lines shown connects the equivalent bromines on of
the two carbons,another connects equivalent methyl groups, and a third connects
equivalent hydrogens (not shown).The meso isomer is just one of the three
stereoisomers of this system. Again, there is one enantiomeric pair plus this
meso isomer, which is achiral. A center of symmetry will be encountered in
any molecule which has two equivalent chiral centers (i.e., both carbons have
the same set of four distinct substituents) and in a conformation of such
a molecule in which all identical groups are anti to one another. The two
carbons of this molecule both have H,methyl,bromine, and 1-bromoethyl substituents.
- Please note that the stereogenic center need not be carbon.
It can be a quaternary nitrogen atom ( the nitrogen of an ammonium salt, if
there are four different groups attached to the nitrogen.
Symmetry Elements Which Guarantee
Achirality
R,S Nomenclature
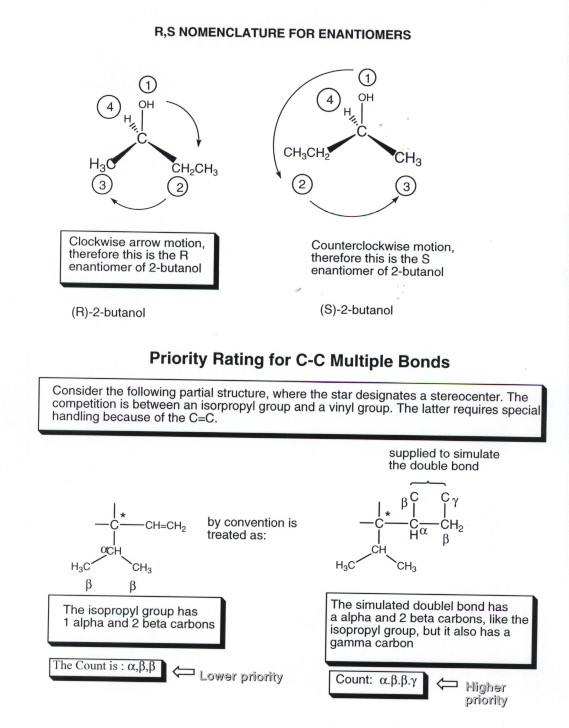
NAMING ENANTIOMERS
Since two enantiomers are different compounds,
we will need to have nomenclature which distinguishes them from each other.
The convention which is used is called the (R,S) system because one enantiomer
is assinged as the R enantiomer and the other as the S enantiomer. What are
the rules which govern which is which??
- Priorities are assigned to each of the four different groups
attached to a given stereogenic center (one through four, one being the group
of highest priority). (It should be understood that each stereogenic center
has to be treated separately.)
- Orient the molecule so that the group of priority four (lowest
priority) points away from the observer.
- Draw a circular arrow from the group of first priority to
the group of second priority.
- If this circular motion is clockwise, the enantiomer is
the R enantiomer. If it is counterclockwise, it is the S enantiomer.
HOW TO ASSIGN GROUP PRIORITIES
There is also a set of conventions
(rules) which govern the setting of group priorities, which is a part of the
R,S system of nomenclature.
- Priority is based upon atomic number, i.e., H has
the lowest priority, O over C, F over O, and so on. Priority assignment
is based upon the four atoms directly attached to the stereogenic center.
For example, in 2-butanol, the example we considered previously, the four
atoms are H,O, and two C's. Oxygen gets the first priority, and H the fourth.
But the methyl and ethyl groups both are attached through carbon , so there
is initially a tie for the second and third priorities.
- In this kind of tie situation, priority assignments proceed
outward to the next atoms, which we will call the beta atoms.(The directly
attached atoms are the alpha atoms). For the methyl group, the alpha atom
is carbon and the beta atoms are three H's, while for the ethyl group the
alpha atom is also carbon and the beta atoms are two H's and 1 carbon. This
beta C of the ethyl group wins the priority competition because there is no
beta atom on the methyl group which has an atomic number greater than 1 (all
three beta atoms are H). In general, the competition contines from alpha to
beta to gamma to delta atoms until a tie-breaker is found.
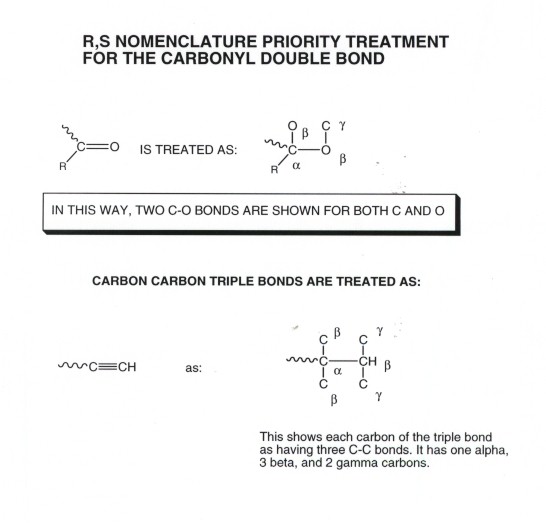
- Some additional conventions are necessary for handling multiple
bonds and aromatic bonds, and these are a little tricky to learn. As an example,
take the vinyl group. Each carbon of this double bond is considered to have
two bonds to carbon, because of the double bond. In the case of a carbonyl
group, the carbon is considered to be bonded to two oxygens, and the oxygen
is considered to be bonded to two carbons. For this reason, a vinyl group
has priority over an isopropyl group, as shown in the illustration.
Two Stereogenic Centers
Non-Equivalent Stereogenic
Centers
- When a molecule has two stereogenic centers, each of them
can be designated as R or S. Thus there are four possible stereoisomers. If
we designate one stereocenter as "a" and the other as "b"
just for labelling purposes, the four stereoisomers can be designated as RaRb,RaSb,SaRb,
and SaSb These designations correspond to the cirucumstance
theat stereocenter "a" can have the R or S configuration ,and stereocenter
"b" can have either configuration.
- In general, if there are n such stereogenic centers , there
will be a maximum of 2n stereoisomers. For example, with three
stereogenic centers, there are eight possible stereoisomers. The maximum of
2n occurs when there are all non-equivalent stereocenters. Stereogenic
centers are equivalent when all four substituents attached to the center are
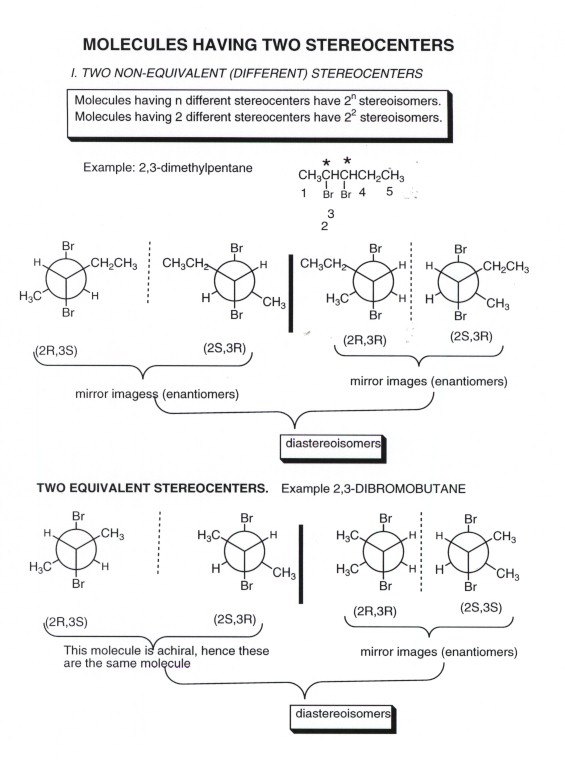
identical. For example, in 2,3-dibromobutane, both stereogenic carbons have
a H, a Br, a methyl, and a 1-bromoethyl substituent. The maximum of four stereoisomers
is not observed here, as we saw before. In fact there are three stereoisomers,
including one achiral stereoisomer. This is because the 2R,3S molecule is
identical to the 2S,3R molecule, since carbons 2 and 3 are equivalent.
- On the other hand, 2,3-dibromopentane has two non-equivalent
stereogenic centers and there are four stereoisomers, consisting of two pairs
of enantiomers. It should be noted that the relationship between one enantiomeric
pair and the other pair of enantiomers is that they are diastereoisomers..
TWO EQUIVALENT STEREOGENIC
CENTERS
- As noted above, when both stereogenic centers are equivalent,
the number of stereoisomers is less than the maximum of 2n, but
in fact is n + 1. In the case of two stereogenic centers (n = 2), there are
3 stereoisomers, as we saw for 2,3-dibromobutane. There is, first of all ,
a pair of enantiomeers: these are the (2R,3R) and (2S,3S) isomers. Note that
the mirror image of 2R,3R is 2S,3S ( i.e., the mirror image inverts the configuration
at each stereocenter).
- There is also an achiral stereoisomer. A molecule which
has stereocenters but is achiral is called a meso compound. We saw
in an earlier diagram that this molecule has a point of symmetry in its most
stable conformation.
- It should be noted carefully that the meso isomer is a diastereoisomer
of the two enantiomers.
COMPARATIVE PROPERTIES OF ENANTIOMERS
AND DIASTEREOISOMERS
DIASTEREOISOMERS
- Diastereoisomers are not mirror image isomers. They
are essentially like any other pair of isomers (e.g., constitutional isomers)
in that they have distinct chemical and physical properties. Since they have
the same functional groups, however, they are usually rather similar to one
another in their reactions and properties.
- Two diastereoisomers can usually be separated from one another
by , e.g., recrystallization, since they have different solubilities.
- Although their chemical properties(reactions) are similar,
the two diastereoisomers will typically react at different rates.
ENANTIOMERS
- Since two enantiomers are mirror images of each other, they
are not distinguished by any physical or chemical means which cannot distinguish
mirror images, i.e., which are not themselves chiral (handed, meaning can
distinguish left from right).
- Therefore 2 enantiomers have exactly the same energy, solubility
in typical achiral solvents, boiling and melting points, NMR and IR spectra,
etc.
- Their chemical properties, including both the qualitative
reactions and the quantitative rates of reaction are identical when reacting
with achiral chemical species.
- In general, then, both chemical and physical properties
of 2 enantiomers are exactly identical twoard achiral agents,chemical or physical.
,li>It is important to realize, however, that when 2 enantiome4s react
with a pure single enantiomer of another chiral compound, the rates of reaction
of the 2 enantiomers will be different (more later).
- Also, one physical property which can distinguish them is
"optical activity" (see below).
OPTICAL ACTIVITY
- Since enantiomers are "handed" or "chiral",
they can be distinguished by other agents which are chiral. Thus, we can easily
tell, in using our right hand to shake hands with another person, whether
that person is using his left or right hand. There is a better "fit"
of the two right hands than there is of right hand to left hand.
- Chemically this occurs, as noted above, when enantiomers
react with another chiral compound. Both the original enantiomer and its reactant
distinguish left from right , so then one of the original enantiomers will
find a better energetic fit with the chiral compound than will the other.
- One physical property which distinguishes 2 enantiomers
is "optical activity". This term refers to the property of chiral
compounds (exclusively) of rotating the plane of plane-polarized light to
the right (clockwise) or to the left (counterclockwise).
- The two enantiomers have exactly the same ability to
rotate this plane, quantitatively, but they rotate it in opposite senses.
Thus, if one enantiomer rotates the plane by 10.5 degrees clockwise (considered
a positive rotation), the other rotates it by -10.5 degrees (i.e., in the
counterclockwise direction).
- Since the exact amount of the rotation of the plane by a
given enantiomer depends upon how much of that enentiomer the light encounters
as it passes through the solution, the measured rotation is divided by the
concentration of the enantiomer and by the path length of the polarimeter
cell to give a true measure of the inherent ability of the enantiomer to rotate
the plane of polarized light. This number is called the specific rotation.
Note that in deriving the specific rotation, the concentration is taken in
grams per mL, and the path length in decimeters. The magnitude of the rotation
also depends upon the wave length of the plane polarized light, so the a single
wave length is usually used, i.e., the sodium D line (529 nm),the line responsible
for the yellow color of sodium-vapor lamps.
- A positive (clockwise) rotation is sometimes called dextrorotation
and a ngetaive rotation is sometimes called levorotation
RACEMIC MIXTURES
- A racemic mixture is a 50:50 mixture of the 2
enantiomers of a chiral compound.
- Because the two enantiomers have equal and opposite specific
rotations, a racemic mixture has a specific rotation of zero, i.e.,
it is optically inactive
- In nature, most naturally occurring compounds occur as
a single enantiomer, not as racemic mixtures. The importance of racemic
mixtures is that ordinary laboratory synthesis which generate a stereogenic
center produce a racemic mixture. For example,if 1-butene is converted
to 2-butanol by the addition of water catalyzed by acid, a stereogenic center
is created in a molecule where none previously existed. Since both enantiomers
have equal energy, and since there is nothing in the catalyst or solvent or
reactant that is chiral, both enantiomers are formed in equal amounts(for
a mechanistic explanation, see later).
- Whereas racemic mixtures are not particularly desirable,
they are not problematic in many labaoratory organic syntheses. However, in
the manufacture of drugs, usually only a single enantiomer is effective, so
that it is desirable to synthesize only a single enaniomer. Nevertheless,
racemic drugs are often used anyway because the other enaniomer is harmless,
and racemic mixtrues are easier(read, cheaper) to synthesize.
OPTICAL PURITY
- If the specific rotation of a pure single enantiomer is
known, it is easy to determine the purity of a sample containing both enantiomers
in unequal amounts. The %OPTICAL PURITY = specific rotation of the sample/specific
rotation of the pure enantiomer. This particular measure of optical purity
is often called ENANTIOMERIC EXCESS( or ee) because it gives %R - %S. A small
problem (admittedly very small, mathematically) arises in converted the ee
(enantiomeric excess) into a specific composition given in terms of %R and
%S. One simple way of doing this is as follows: If the enantiomeric excess
of the R enantiomer is, for example, 80%, this means that there is 80% of
the R enantiomer plus 20% of the racemic mixture (not 20%S). Since the racemic
mixture is 10%R and 10%S, the composition of the mixture is 90% R and 10%S.
Remember: ee represents not the % of one of the enantiomers, but the difference
between the % of one pure enaniomer and the % of racemic mixture).
SEPARATION OF ENANTIOMERS
- The separation of 2 enantiomers present in a racemic
mixture or any mixture of enantiomers, is called resolution .
- Enantiomers are not readily separated by conventional
means, such as recrystallization or fractional distillation, since they
have the same solubilities, m.p.'s, b.p.'s, etc. So, special means are required
for "resolution" of two enantiomers.
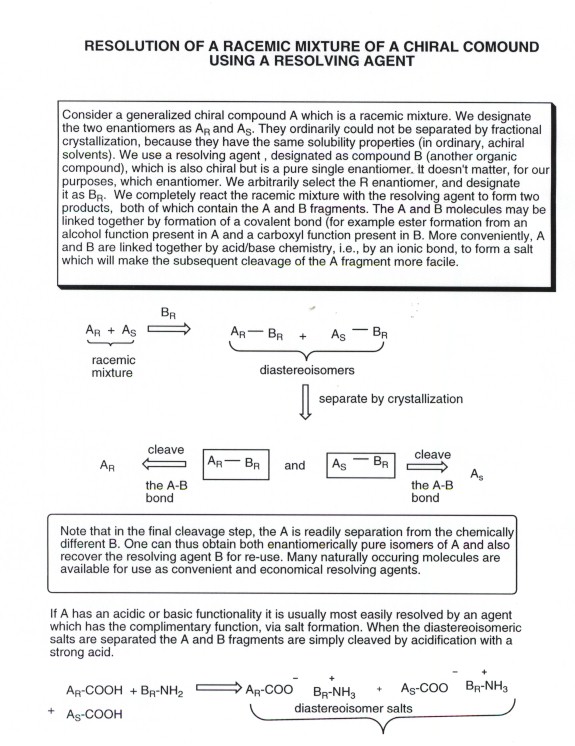
- One common strategy for resolution is often to take advantage
of the circumstance that, while enatiomers have the same solubilities and
cannot be readily separated by simple recrystallization, diastereoisomers
have different solubilites. The two enantiomers present in a racemic mixtrue
can be reacted with a pure enantiomer of a chiral compound (called a resolving
agent) which we have on hand (many occur in pure form in nature). This will
form a compound with two chiral centers, and will give rise to 2 different
diastereoisomers which can be separated from each other. Following this
separation the chiral resolving agent rcan be removed by through some chemical
reaction to give the two separate enantiomers. The chiral resolving agentcan
also be recovered for re-use.
- As an example, consider the generalized case shown in the
illustration below.
KINETIC RESOLUTION USING ENZYMES
- Enzymes are proteins which have many chiral centers and
which occur in nature as a single enantiomer (out of all the myriads of possible
stereoisomers).
- The rates of reaction of two enantiomers with a single enantiomer
of any chiral substance are different. We can see that the products will be
diastereoisomeric, and so of different energies, and the rates of formation
of these products will in general be different. In other words, a "handed"
molecule can distinguish chemically between 2 mirror image isomers. Enzymes
are particularly effective in making this distinction, so that a racemic mixture
can often be easily resolved by reaction with some simple substance in the
presence of the chiral enzyme as catalyst. The enantiomer whiich reacts faster
will be converted to a new compound having an entirely different functional
group, while the enantiomer which reacts more slowly will remain unreacted.
The separation of the two compounds is then quite easy.
- As an example, if the compound which is the racemic mixture
has an alochol function, it can be converted to an acetate ester by reaction
with acetic acid in the presence of a suitable esterifying enzyme. The separation
of the ester of one enantiomer from the alcohol of the other is then very
easy. Then ester can then be hydrolyzed to the alcohol, if desired, by either
simple chemical means or by enzyme catalyzed reaction.

RETURN TO THE TOP OF THIS PAGE
ON TO THE NEXT CHAPTER:ALKENES
I
BACK TO THE PREVIOUS CHAPTER
BACK TO THE BAULD HOME PAGE








