TOP
ALKENES I:PROPERTIES OF ALKENES
TABLE OF CONTENTS FOR THIS
CHAPTER
- Structure and Hybridization of Ethene
and other Alkenes
- Cis/Trans Isomerism in Alkenes
- Alkene IUPAC Nomenclature
- The E,Z System of Alkene Nomenclature
STRUCTURE AND HYBRIDIZATION
OF ETHENE AND OTHER ALKENES
- Recall that when carbon is bonded to only three other
distinct atoms, it is normally sp2 hybridized.
Please review : The Hybridization Unit
,the development of this hybridization state, the sigma framework of ethene,
the pi bond, and other aspects of the bonding in ethene which were discussed
early in the semester. You will need to know these very well for this unit
and the corresponding exam, and be able to explain them and illustrate them
pictorially.
- You should know why ethene is fully planar (it uses
only 2px and 2py atomic orbitals, and has no z component.
So all orbitals are oriented in the xy plane.
- You should know why the bond angle is approximately 120
degrees (there are three approximately equivalent hybrid orbitals in the xy
plane. The full circle of 360 degrees is thus divided into three approximately
equivalent angles. We use the term "approximately" here, because
the angles are not exactly equivalent and are not exactly 120 degrees. This
is because the atoms to which carbon is bonded are not the same (2H's and
1 C), therrefore the three sigma bonds are not exactly equivalent. The HCH
angle is less than 120 and the HCC angle is greater than 120.
- You should know that the C=C is comprised of one sigma and
one pi bond, and that optimum pi overlap requires the two 2pz orbitals
to be "parallel", i.e., to have a 0 degree dihedral angle for the
most efficient overlap. Recall that pi overlap is lateral and thus is less
efficient than sigma overlap. Hence the pi bond is weaker than the sigma bond,
easier to break, making alkenes much more reactive than alkanes. You
should know that the pi bond strength is ca. 63 kcal/mol, and that the pi
bond is completely broken by a relative rotation of 90 degrees.
- You should also recall that rotation around the sigma
bond of ethene by 90 degrees, completely breaks the pi bond. This is because
two perpendicularly oriented 2p AO's have equal amounts of positive (bonding)
and negative (antibonding) overlap. The net overlap is therefore precisely
zero, even though there are regions of overlap of the two orbitals. This 90
rotation therefore requires an expenditure of energy of ca. 63 kcal/mol, and
is therefore extremely difficult.
Cis/Trans(Geometric)Isomerism
- Since rotation around the C=C is strongly resisted by the
pi bond, as noted above, alkenes which have a substituent on both carbons
of the double bond can exist as two different isomers in which the substitutents
are either on the same side of the double bond (cis) or opposite sides (trans).
These are called cis/trans isomers or geometric isomers. You should also understand
that cis/trans isomers have the same connectivity, so that they are stereoisomers.
Further, they are clearly not mirror images (the alkene is achiral), so that
they must by of the type called diastereoisomers.
- The simplest case is that of 2-butene, in which the methyl
groups are cis or trans on the double bond (see illustration). These two compounds
cannot be interconverted without breaking the pi bond, so that they are not
simply different conformations (conformations refer to different structures
generated by rotation around a single bond, which is easy to do).
- The trans isomer is exactly 1.0 kcal/mol more stable than
the cis isomer because of a steric effect. That is, in the cis isomer,
one of the hydrogens on one methyl group is closer to a hydrogen on the second
methyl group than the sums of the van der Waals radii of two hydrogen atoms.
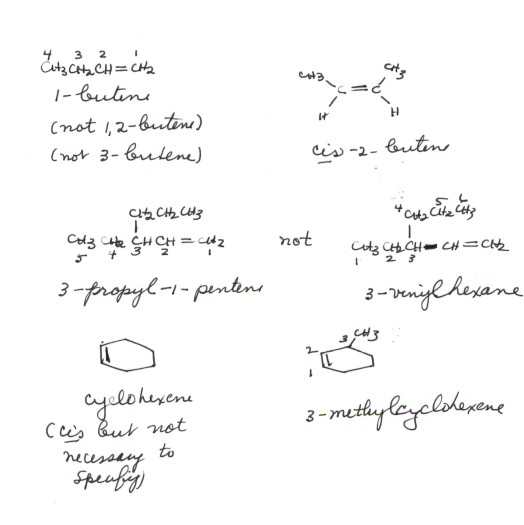
- You should note that none of ethene, propene, or 1-butene
have cis/trans isomers.

RELATIVE THERMODYNAMIC STABILITIES
OF ALKENES
- The relative thermodynamic stabilities of various alkenes
can be determined by heats of hydrogenation. For example, the reactions
of 1-butene and both cis- and trans-2-butene with dihydrogen afford the same
product, butane. Consequently, differences in the heats of hydrogenation accurately
reflect the differences in thermodynamic stabilities of these three alkenes.
These heats are: 1-butene, -30.3; cis-2-butene, -28.6; trans-2-butene,
-27.6 kcal/mol. The order of stabilities is therefore 1-butene least stable
and trans-2-butene most stable (recall that the more heat that is liberated,
the less stable the reactant). The stability difference between 1-butene
and trans-2-butene is 2.7 kcal/mol. Recall that it takes only 1.37 kcal/mol
to make a difference of a factor of ten in the equilibrium constant for a
reaction at room temperature.
- There are two factors which affect the stability order.
First, the presence of alkyl groups directly attached to the double bond
tends to stabilize the system. We regard 1-butene as a monoalkylsubstituted
double bond, since it has one methyl group attached to the double bond. In
contrast, the 2-butenes have two methyl groups attached to the double bond.
These disubstituted double bonds are therefore more stable than the
monosubstituted double bond of 1-butene.
- The second factor is relevant to the relative stability
order of the two disubstituted double bonds, i.e., cis- and trans-2-butene.
The methyl groups of the cis isomer are relatively close in space, so that
a steric repulsion of 1.0 kcal/mole results, as was mentioned previously.
The trans isomer, which has no such steric effect, is therefore the more
stable isomer.
- It is interesting and significant the the heat of hydrogenation
of any 1-alkene, such as 1-pentene or 1-hexene is essentially identical to
that of 1-butene, i.e., -30.3 kcal/mol. So any monosubstituted alkene
has essentially the same thermodynamic stability. Note the marked difference
between heats of hydrogenation and heats of combustion. In the latter case,
we cannot compare molecules which are not isomeric, because the entire molecule
(all bonds) is being broken down, and the larger molecule provides more fuel
for combustion. However, in hydrogenation, only the double bond is being affected,
so we can essentially (it is an approximation, but a good one) assume that
hydrogenation heats reflect only the relative stabilities of the alkene
pi bonds, even for non-isomeric alkenes.
- We should note also that the heat of hydrogenation of ethene
itself is larger than for any mono- or disubstituted alkene. Thus, ethene
is the least thermodynamically stable of the simple alkenes. The heat
of this hydrogenation is -32.8 kcal/mol, which is 2.7 kcal larger than for
the monosubstituted alkenes. So, the first methyl group provides ca. 2.7
kcal/mol of stabilization to the pi bond, while the second provides exactly
the same amount (difference in heats between 1-butene and trans-2-butene).
- Please note that trisubstituted and tetrasubstituted
double bonds are even more stable (liberate les heat upon hydrogenation) than
disubstituted double bonds.
- You should be able to examine a given alkene structure and
identify the alkene as a mono-, di-, tri-, or tetrasubstituted alkene. Note
that ethene has 4 hydrogens attached to the double bond. A monosubstituted
alkene has 3, a disubstituted alkene has 2, a trisubstituted alkene has 1,
and a tetrasubstituted alkene as none. Please note, also, that the nature
of the alkyl group makes no difference. Essentially any alkyl group
will provide ca. 2.7 kcal/mol of stabilization, whether it is a simple methyl
group, an ethyl group, an isopropyl group, a tert-butyl group, or anything
else. The only factor of energetic significance is whether the C-C double
bond has 0,1,2,3, or 4 alkyl groups directly attaced to it.
- We will briefly discuss the basis for this modest alkyl
group stabilization of an alkene double bond in class.
ALKENE NOMENCLATURE
- The IUPAC nomenclature for alkenes is analogous to that
for alkanes with a very few essential modifications.
- The first modification is that the suffix is no longer the
-ane of an alkane, but -ene. The suffix "ene" in organic
chemistry always refers to a carbon-carbon double bond, i.e., an alkene function.
- The second change is that the numbering of the parent
(longest continuous) chain always begins at the end of the parent chain closest
to the double bond, so that the double bond has the smaller locant.
The position of the double bond in the parent chain must be specified,
but we do not specify the position of the double bond using the position numbers
of both carbons, but just of the first carbon. Thus 1-butene and not
1,2-butene. The use of the 2 would be redundant, because the second double
bond carbon must necessarily follow the first in the chain.
- The third main change is that the alkene function must
always be a part of the main chain , not a side
chain. Even if there is a longer continuous chain, if that chain doesn't contain
both alkene carbons it is rejected as a parent chain.
-
- Cis and trans isomers must be designated by
the appropriate cis- or trans- prefix.
-
- Cycloalkenes are named by using the prefix "cyclo"
before the name of the alkene. Thus, cyclohexene. Note that we do not have
to specifiy 1-cyclohexene, because all carbons are equivalent in cyclohexane,
so that whichever positions the double bond occupies automatically become
the 1 and 2 positions. Number in a substituted cyclohexene then proceeds in
the direction in which the nearest substitutent is encountered.
THE E,Z SYSTEM
There is sometimes a need for a more formal system of nomenclature
for geometric isomers, especially when the cis or trans substituents are not
identical, as they were in the case of the 2-butenes. In such a case, the cis/trans
nomenclature can become ambiguous, whereas the goal of systematic nomenclature
is to have a single, unequivocal name for each organic compound.
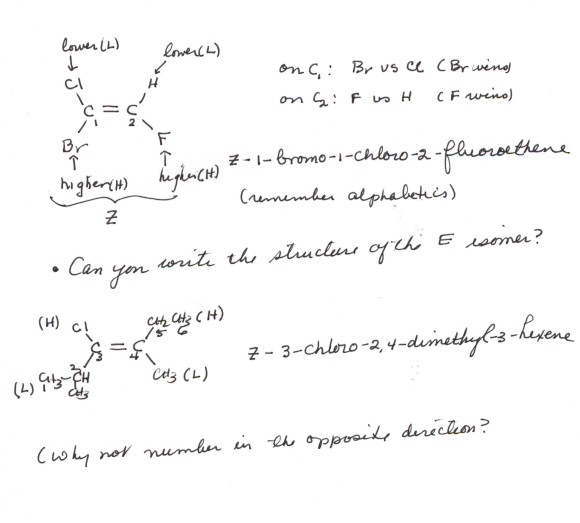
- In the E,Z system of alkene nomenclature, a priority
system is used to rank substituents on the double bond. There are four such
substituents (including the H's ), two on each carbon of the double bond.
The priority system is identical to that used in the R,S nomenclature of stereoisomers.That
is, it is based fundamentally upon atomic numbers. You should review the
priority system. It is also illustrated
below in some examples.
- The procedure is simply to look at each one of the two alkene
carbons in turn, and rank the priority of the two substituents upon that carbon
as higher or lower. The isomer in which the two higher priority groups are
cis is called the Z isomer (from the German word zusammen =
together); if the high priority groups are trans, the isomer is called
the E (entgegen) isomer. Why not call them cis or trans
based upon the priorities?? Good question.
BACK TO THE TOP OF THIS PAGE
ON TO ALKENES 2
BACK TO THE PREVIOUS CHAPTER ON
STEREOCHEMISTRY
BACK TO THE BAULD HOME PAGE
END OF CHAPTER 4



