Butane Conformational Analysis
TOP
TABLE OF CONTENTS FOR THIS PAGE
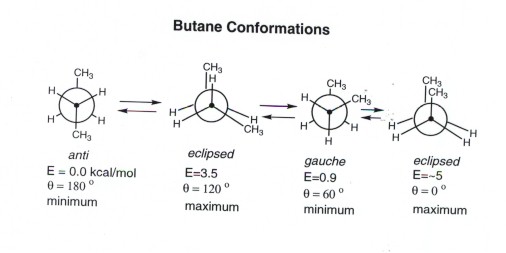
Butane
Conformational Energy Diagram
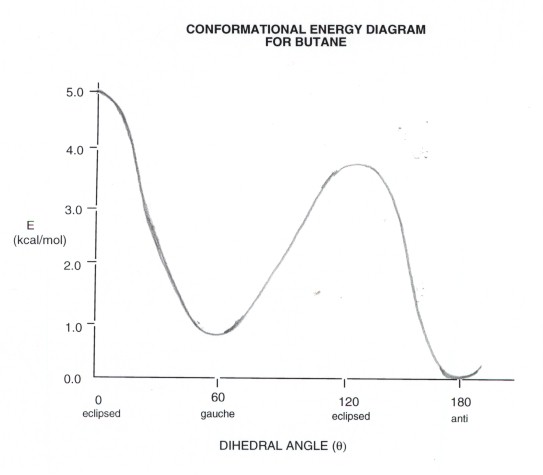
- There are two energy minima, the gauche and anti
forms, which are both staggered and thus have no torsional strain.
- The anti form is the absolute energy minimum,
since the gauche form has a small steric interaction between the two
methyl groups. At a dihedral angle of 60 degrees, one hydrogen of each of
the methyl groups is relatively close to a hydrogen of the other methyl group
(van der Waals repulsion). Carefully note the difference between steric
strain and torsional strain. The latter arises from eclipsed bonds,
while the former arises from atoms which are too close to each other.
- There are also two energy maxima, both of which are eclipsed
and thus torsionally strained. The higher energy conformation also has steric
strain.
-
You should be able to draw these conformations
and this diagram and attach the appropriate numerical energies to each conformation.
You should also be able to identify the type or types of strain present
in each conformation and explain the basis of that strain.
Torsional
strain arises from the repulsion of electrons in bonds; steric
strain arises from atoms that are too close!
BACK TO THE TOP OF THE PAGE
ON TO THE NEXT PAGE
BACK TO THE BAULD HOME PAGE


