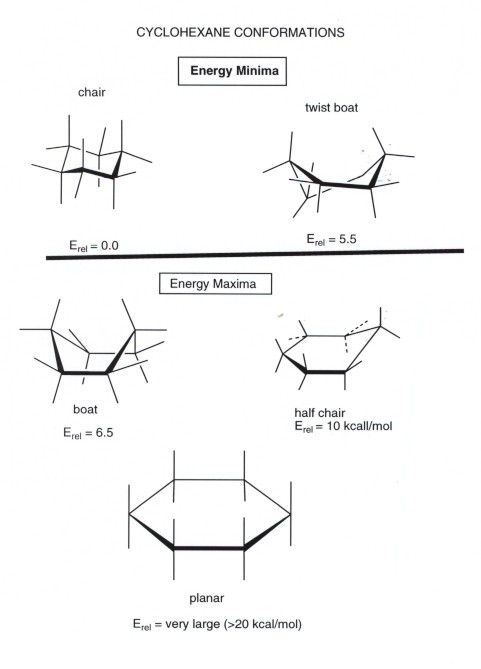
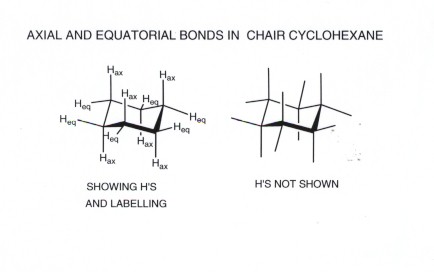
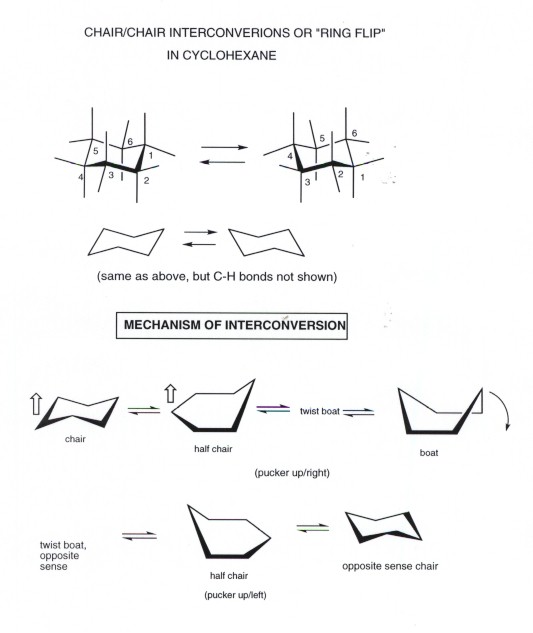
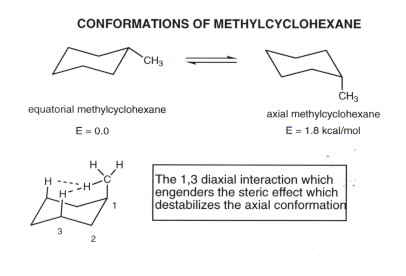
The key difference between the 1,2
and 1,4 patterns, is that in the diequatorial 1,2 conformation, the
methyl groups are gauche as in gauche butane (remember that gauche
essentially implies a 60 degree dihedral angle ). Consequently, this
diequatorial conformation no longer is strain free, as was the 1,4-trans
diequatorial conformation, where the methyls are very far apart. The
1,2-diequatorial isomer is 0.9 kcal/mol less stable than the 1,4-diequatorial
isomer, because of this gauche butane-like interaction (recall that
the gauche isomer in butane is destabilized by exactly this amount).