TOP
Ring Strain in Cyclopropane
The Lack of Ring Strain in Cyclohexane
The Large Ring Strain in Cyclopropane
- Unlike acyclic hydrocarbons, cyclic hydrocarbons may have
strain which is inherent in their cyclic structures. This is called RING
STRAIN.
- Ring Strain consists primarily
of two diffferent kinds of strain: (1)torsional strain and (2)angle
strain.Torsional strain, of course, arises when bonds are not ideally
staggered. In most ring systems, it is impossible to perfectly stagger all
bonds. In cyclohexane, fortuitously, perfect bond staggering occurs in the
chair conformation, but no conformation in any other ring allows for such
perfect staggering.
- ANGLE STRAIN arises when the C-C-C bonds of the ring
depart (because of geometric necessity) from the ideal tetrahedral angle preferred
for sp3 carbon. Again, in chair cyclohexane this angle just happens to be
virtually identical to the tetrahedral angle, so that neither angle nor torsional
strain occurs in cyclohexane in the chair form. Therefore the total ring
strain is nil for cyclohexane.
- Cyclopropane, the smallest
cycloalkane, is rather highly strained (although it is still easily isolated
and stored). The estimated total ring strain in cyclopropane is 28 kcal/mol
(from heats of combustion measurements). When this value is compared with
the strength of a typical C-C bond (ca. 88 kcal/mol), it can be seen
that ring strain substantially weakens the C-C bonds of the ring. Hence,
cyclopropane is much more reactive than acyclic alkanes and other cycloalkanes
such as cyclohexane and cyclopentane.
- The carbon framework of cyclopropane is planar, by definition.That
is , it could not be otherwise, because it takes three points to define a
plane, and the positions of the three carbon nuclei define these points. Thus,
there is essentially no conformational analysis required for cyclopropane.
You might recall that we had earlier pointed out the strain in planar cyclohexane
is large, because all of the C-C bonds are eclipsed when the carbon
framework is planar. This is also true in cyclopropane, or in any other planar
conformation of a cycloalkane. Thus, planar conformations are usually disfavored,
but no other conformation is available to cyclopropane. Since there are three
C-C bonds in cyclopropane, and each of them is staggered, we can estimate
that there is at least 3X3 = 9 kcal/mol of torsional strain in cyclopropane.
- Since the total ring strain in cyclopropane is about 28
kcal/mol, it is evident that the major part of the ring strain must arise
from another factor. This is angle strain. Angle strain in cyclopropane
arises from the circumstance that the C-C-C angle is forced to be 60 degrees
(that of an equilateral triangle), about 49 degrees less than the ideal angle
for the strongest C-C bond (the strain angle is ca. 49 degrees.
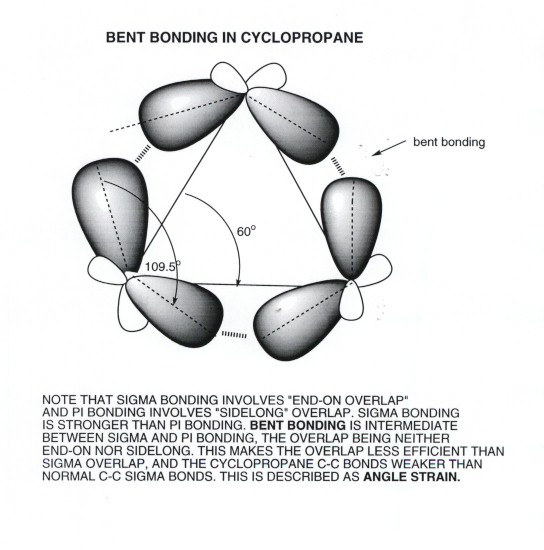
- Looking at this in a little more detail, we can see that
the C-C bonds of cyclopropane are "bent" and not pure
sigma bonds. Recall that overlap is greatest when orbitals overlap "end-on",
i.e., via sigma bonding. Pi bonds overlap laterally. The overlap in cyclopropane
is neither end-on or lateral, but in between. So it is intermediate between
sigma and pi bonding. These bonds are also sometimes called "banana
bonds".
- The cause of the bent overlap is that the internuclear
angle ( the angle between the bond lines connecting the nuclei) is forced
to be much smaller (60 degrees) than the interorbital angle (the angle
between the axis of the two AO's on a given carbon), which is 109.5 degrees.
BACK TO THE TOP OF THIS PAGE
ON TO THE NEXT CHAPTER
BACK TO THE BAULD HOME PAGE
