Ethane Conformational Analysis
TABLE OF CONTENTS FOR THIS
PAGE
Definition of Conformations
Definition: CONFORMATIONS--Different
spatial arrangements (structures) of a molecule which are generated by the relatively
easy rotation around a single bond (usually a C-C single bond).
Things to recall:
-
Single bonds are sigma bonds,
which by definition are symmetrical with respect to the bond axis. Thus
rotation does not change the extent of overlap and thus does not change
the strength of the bond. Consequently rotation around a sigma bond is
relatively easy , in contrast to a pi bond, where rotation around the
bond completely destroys the pi bond.
-
The rotation we're speaking of is "internal
rotation", that is the rotation of one part of the molecule relative
to the other. For example in ethane, the rotation of one methyl group
relative to the other.The angle of rotation is called a dihedral angle
, and it is represented by a Greek theta symbol.
-
Since the different structures which
are generated by this rotation are all very similar (they have the same
number and kind of bonds) and are very easily interconverted, they are
not referred to as isomers but as different conformations of the
same molecule. These conformations have similar energies, but not precisely
the same energy.
-
Since rotation around the C-C bond
of ethane is continuous, there are an infinite number of conformations,
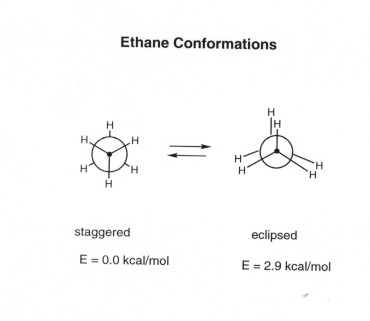
but only two are of major interest. These are the lowest energy
structure, called the staggered conformation, and the highest energy
structure, called the eclipsed conformation.
-
The staggered structure is important
because, being the structure of lowest energy, it is the structure actually
adopted by ethane (the ground state structure). It defines the shape
of ethane molecules. The eclipsed structure is important not because it
is highly populated , but because its energy relative to the staggered
structure defines how much energy is required to complete a rotation in
ethane. This is called the energy barrier to rotation, and it is
about 3 kcal/mol. That is, the eclipsed structure is 3 kcal higher in
energy than the staggered structure.
Torsional Strain
-
Torsional Strain
-
An energy increase caused by the eclipsing
of bonds.
-
Strain
-
Any increase in energy of a molecule,
particularly an increase relative to that which would normally be expected.
-
Eclipsing
-
The relationship between two bonds when
the dihedral angle is zero.
-
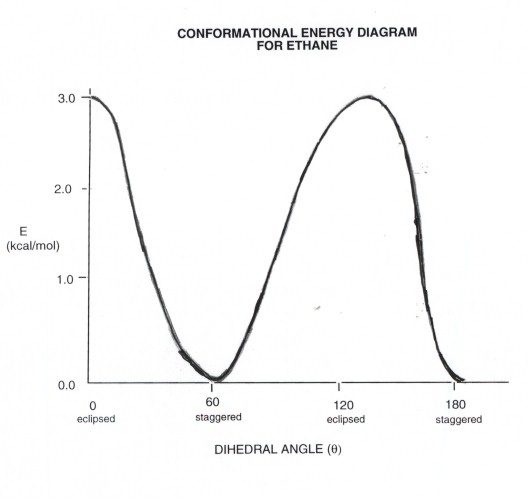
Conformational Energy
Diagram
-
A plot of energy,E (vertically) vs. dihedral
angle,theta (horizontally). It should include the structures,energies,
and names of the energy minima and maxima. You should be able to draw
such a diagram for ethane.
Conformational
Energy Diagram for Ethane
Basis
for Torsional Strain
TORSIONAL STRAIN
IS CONSIDERED TO BE CAUSED BY THE REPULSIONS BETWEEN ELECTRONS IN THE TWO
BONDS WHICH ARE ECLIPSED. THESE ELECTRONS ARE CLOSER TOGETHER IN THE ECLIPSED
FORM THAN IN THE STAGGERED FORM, AND SO THE REPULSIONS ARE GREATER. RECALL
THAT REPULSIONS BETWEEN OPPOSITELY CHARGED PARTICLES REPRESENT AN INCREASE
IN POTENTIAL ENERGY.
Conformational Analysis of Ethane
Conformational Analysis
It is important to note that in ethane there
are three sets of bond eclipses taken pairwise. Since the total torsional
strain is 3 kcal, it is usually considered that each pair of bond eclipses
engenders 1 kcal of torsional strain. This kind of analysis is useful
for estimating the torsional strain present in other, more complex, molecules,
as we shall see and in understanding the ground state structures and shapes
of molecules, which we call conformational analysis.
Newman Projection
Structures
Newman Projection Representations
You should be able to represent the staggered and eclipsed
structures of ethane using Newman Projection Structures as shown above.
ON TO THE NEXT PAGE (BUTANE
CONFORMATIONAL ANALYSIS)
BACK TO THE BAULD HOME PAGE

