CHAPTER 2: VOLUME 1
Alkanes
CONTENTS OF THIS PAGE
C-H Bonds.The Simple Alkanes:
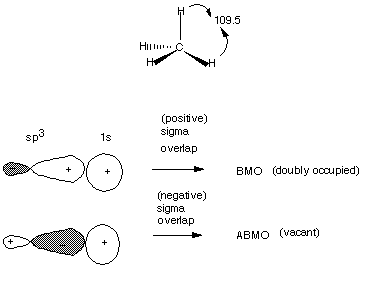
The simplest alkane is methane, which contains one carbon atom. Its molecular
formula is CH4. It has four equivalent C-H bonds, formed by the overlap
of the four carbon sp3 hybrid orbitals with the 1s AO of four different
hydrogen atoms. The bond angle is the tetrahedral angle, 109.5 _. The structural
representation of methane is given below, using the convention that bonds
which project toward the observer are emboldened and broaded toward one end,
bonds which project away from the observer are hashed, and bond which neither
project away from nor toward the observer (are in the plane of the writing surface)
are normal lines. The scheme below also illustrates the BMO and the
ABMO of one C-H sigma bond. Remember that this is a sigma bond .

C-C Bonds; General Formula of Alkanes.
The next simplest alkane is ethane, C2H6. In general the alkanes have the formula CnH2n+2, where n = the number of carbon atoms in the alkane.Ethane has six C-H bonds very similar to those in methane, but in addition, it is the simplest alkane which has a C-C bond. This is also a sigma bond formed by the overlap of sp3 orbitals on each carbon. There are two different bond angles, HCH and HCC, but both are very close to the tetrahedral value. You should learn the names of the first 10 alkanes: methane, ethane, propane, butane, pentane, hexane, heptane, octane, nonane, and decane.Except for the C1-C4 alkanes, the prefixes are numerical and easily remembered. All alkanes have the commone suffix -ane. You should be able to draw line drawings and condensed structures for all of these alkanes. Please note that the -CH3 structural feature in the condensed structures is called a methyl group, and the -CH2 is methylene. (CH3CH3, ethane; CH3CH2CH3 = propane etc) Note also that all of these hydrocarbons have methyl groups at each end, and are connected by various numbers of methylene groups. They are referred to as a homologous series, because they each member differs from the next in the series by a constant, CH2.
Illustrations of Constitutional Isomers:
1. Ethanol and dimethyl ether both have the molecular formula.C2H6O. However, ethanol has the structure CH3CH2OH, while dimethyl ether has the structure CH3-O-CH3. Thus the connectivity of the former is C-C-O while that of the latter is C-O-C.
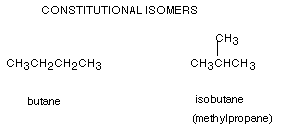
2. BUTANE: The C4 alkane is called butane, having the molecular formula C4H10. However, their are two constitutional isomers having this formula, so one isomer is called butane and informally, the other is called isobutane. We shall shortly learn to name that latter compound according to IUPAC rules, rather than by adding prefixes to the name butane. When we come to pentane, there are 3 isomers, and the number of isomers increases rapidly as the number of carbons increases. Therefore it is essential to have a systematic method of naming these compounds.
C5 --- 3 isomers:
C6----6 isomers
C10--75 isomers
C30---4,111,846,763
CONCLUSION: THE USE OF PREFIXES TO NAME EACH SPECIFIC ISOMER IS HOPELESS!
IUPAC (Systematic) Nomenclature
Consider the following two molecules which are constitutional isomers and use them to illustrate the IUPAC approach to nomenclature:
![]()
The first molecule is hexane, but how to name its constitutional isomer?
Step 1: Locate the longest continuous chain
of carbon atoms and name the branched isomer as a derivative of this with the
appropriate substituents in the appropriate places. Thus the isomer above is
named as a derivative of pentane. (Of course it is not pentane, itself). Pentane
is called the "parent name".
Step 2: Number the chain beginning with the
end of the chain nearest the first substituent (branch). In the drawing
above, the left hand end (terminus) is nearest to the methyl side chain, so
numbering goes from left to right, instead of right to left.
Step 3: Place the names and position numbers (locants) of any substituents before the parent name. The last substituent listed is joined to the parent name without any separator (hyphen, comma, etc.), but position numbers are separated from substituent names by hyphens. Thus the name of our compound is 2-methylpentane. (There is also a 3-methylpentane as well as several dimethylbutanes which are isomers of hexane. See if you can draw these. A good procedure is just to draw out the skeleton connectivities and then fill in the requisite number of hydrogens for each carbon).
A CAVEAT (WARNING) CONCERNING THE SELECTION OF THE PARENT CHAIN (THE LONGEST CONTINUOUS CHAINI): EXAMINE ALL POSSIBLE CHAINS, NOT MERELY THE HORIZONTAL ONE (THE ONE WRITTEN ON LINE).
Substituents or groups like CH3- are essentially alkanes having one hydrogen atom removed and replaced by an unspecified valency. Thus the methyl group is methane minus one hydrogen, with the unspecified valency (hyphen or line) added. To name a group or substituent, simply delete the family suffix of an alkane (-ane) and replace it with the family name for a substituent or group (-yl). Thus, the CH3CH2- substituent is ethyl, etc.
(1) If two different chains of equal length are present,
choose the one as parent which has the larger number of branches (substituents).
This will mean the substituents are smaller than for the other possible choice.
Example:
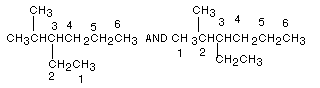
The numbering system in the second drawing is the correct
one. The correct name is:
3-ethyl-2-methylhexane.The incorrect numbering system would have lead to the incorrect name 3-isopropylhexane.
IMPORTANT NOTE: WHEN THERE IS MORE
THAN ONE SUBSTITUENT, THE SUBSTITUENTS ARE LISTED IN ALPHABETICAL ORDER,
not arranged according to the numbers of their respective positions (see above:
3-ethyl-2-methyl not 2-methyl-3-ethyl).
(2) There is also a tie-breaker for numbering the parent chain.
If a first substituent occurs equally close to either terminus of the parent
chain, the nearness of the second substituent to the termini of the chain is
determinative (and so on, to the third or fourth substituent, if necessary).
Example:
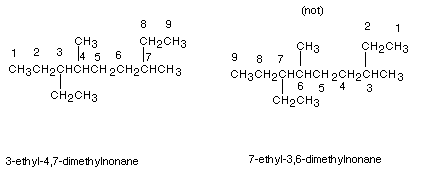
Note that in the first numbering system, the first substituent when numbering from left to right is an ethyl group at the 3 position; also when numbering from right to left (second structure) the first substituent is encountered at the 3 position also (methyl). A tie-breaker is therefore needed. In the left to right numbering, the second substituent is encountered at the 4 position (methyl) , while in the right to left numbering, the second substituent (also methyl) is at the 6 position. So the left to right system is chosen.
Complex Substituent Nomenclature.
Substituents other than methyl, ethyl, propyl, butyl etc. are also named preferably using IUPAC systematic nomenclature. The rules are essentially the same as for naming alkanes except for the following: (1) Numbering in the parent chain of the substituent begins at the carbon atom having the unspecified valence (2) the family suffix for a substituent , as noted earlier, is -yl. Examples of the IUPAC naming of a number of substituents are given below. Also given are the "common" or "trivial" names of the more common substituents.
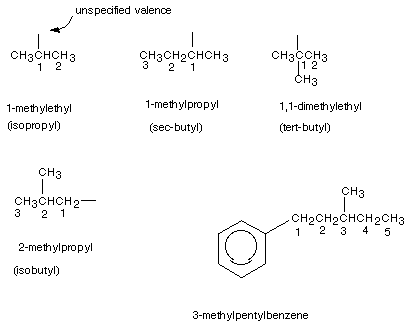
PRIMARY, SECONDARY, TERTIARY, AND QUATERNARY CARBON ATOMS
Primary carbons, symbolized as 1 o(the degree sign should be superscripted as in the drawing below) carbons, are carbons which are attached to only one other carbon atom. In an alkane, these are methyl groups. Secondary carbons, symbolized as 2 o carbons, are carbons which are attached to two other carbon atoms. In alkanes, these are methylene groups. Tertiary carbons , symbolized as 3 o carbons, are carbons bonded to three other carbons. These are methine carbon atoms (CH) in alkanes. Quaternary carbons are carbon atoms which are directly bonded to four other carbon atoms. These types of carbons are all illustrated in the example given below:
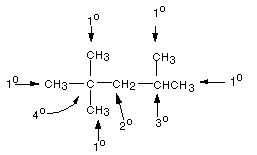
YOU SHOULD BE ABLE TO CLASSIFY ANY CARBON ATOM FOUND IN A STRUCTURE AS ONE OF THESE TYPES
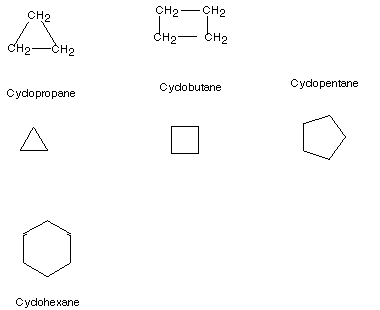
Cycloalkanes are alkanes in which the carbon skeleton
is cyclic. Examples of these are shown below. Basically any ring size, even
including very large sizes, is possible. The general formula is CnH2n,
since there are two less hydrogen atoms in these compounds than in acylic
alkanes . Note that they consist solely of methylene groups, having no terminal
methyl groups as do alkanes. They are named simply as the acyclic alkane of
the same number of carbon atoms, with the prefix "cyclo" attached
to that name, using no separator.
Substituent Nomenclature is also useful in naming cycloalkanes.
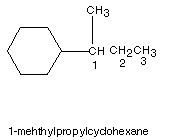
Nomenclature of Substituted Cycloalkanes:
In naming substituted cycloalkanes, some additional rules are needed: