
Class Notes: Volume 2
POLAR COVALENT BONDS: Covalent bonds may be pure covalent, as in the case of dihydrogen or dichlorine or dinitrogen--i.e. they may have zero bond dipole moment (charge separation). Most covalent bonds, however, are at least somewhat polar, because of the electronegativity difference between the two atoms joined by the bond.
Definition: Electronegativity--- the relative tendency of a nucleus of an atom to attract electrons in a covalent bond.
DIPOLE MOMENTS.Thedipole moment is a property of a molecule, not a bond. It is a vector sum of the bond moments of all bonds in the molecule.

For example, the C=O bond is highly polar , with carbon being the positive end, but the molecule carbon dioxide has a zero dipole moment because the two bond dipoles are oriented in exactly opposed directions (carbon is sp hybridized) and their vector sum is zero. In contrast, sulfur in sulfur dioxide has a bent OSO angle and the dipole moments of the bonds do not cancel. Thus, the latter molecule is polar, while carbon dioxide is non-polar.
CAVEAT: Be certain to make the distinction between bond moments and dipole moments. Also, between bond polarity and molecular polarity.
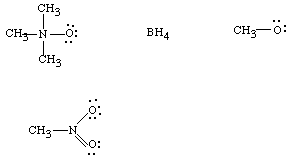
FORMAL CHARGES ON ATOMS: Even in neutral molecules, the best valence structures we can write will necessarily have a unit charge on one or more atoms. That is, by following the normal rules of valence and counting electrons, one sees that certain atoms must have one electron more or less than when they are neutral. In actuality, these charges will rarely be exactly unit charges, so they are called "formal charges". An atom which has a unit formal charge will typically have a very large actual charge, approaching one. Consider the following examples:

In the first example, N which has atomic number 7 and is in groups 5, has 5 electrons in its valence shell when it is neutral. If , for any covalent bond, we partition the 2 electrons equally to each participating atom, N has only 4 electrons in its valence shell--i.e., one from each of the covalent bonds. Thus it must be assigned a formal charge of +1. In reality, this charge will be shared somewhat by the H's, because the N-H bonds will be polar covalent, and N will withdraw electrons to some extent from H ( that is, it will have a partial negative charge from the covalent bond). So we write the ammonium ion has having a formal charge on nitrogen. By the way, H should have 1 electron when neutral, and it does. So the H's do not have a formal charge. The total charge on the molecule is the sum of the formal charges on the atoms , and so is +1 for this species.
Similarly, any tetravalent nitrogen compound will have a formal
charge of +1, so that is true of the N atom in the second example, too. But
what about boron. Well, B has atomic number 5 and a total of 5 electrons, but
two of these are not in the valence shell. It should have 3 valence shell electrons
when neutral (corresponding to Group 3 in the Periodic Table). But how many
electrons does it formally have? One from each of the BH bonds and one from
the BN bond (one from any covalent bond).
In general, you can calculate formal charges from the following equation:
FC(Formal Charge) = Group number - Number of Covalent Bonds- Number of unshared electrons.
For example, for B in the above example: FC = 3 - 4 = -1. So boron has a formal charge of -1 and N a formal charge of +1. The overall charge of this chemical species is therefore zero.
Net Charge on the Species = Sum of Formal Charges of all atoms.(You can always assume that H has a formal charge of 0 unless it is the proton or hydride ion.) You should be able to calculate formal charges on all atoms in the following cases as exercises and also to calculate the overall charge on the chemical species. Assume that Carbon and Hydrogen have zero net charges ( you should also be able to verify this).
Resonance Structures and Resonance Theory
RESONANCE STRUCTURES AND RESONANCE THEORY: There is a special tutorial on this web site on the very important subject of Resonance Structures and Resonance Theory. Be sure that you know the rules for writing resonance structures and for qualitatively determining the extent of resonance stabilization.
END: CHAPTER ONE
BACK TO THE PREVIOUS PAGE OF NOTES