Note that both structures have the same number and kinds of bonds and therefore must be equal in energy.
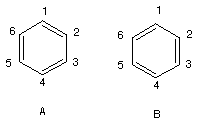
Consider the two possible valence structures A and B for benzene:
Note that both structures have the same number and kinds of bonds and therefore must be equal in energy.
In order to be able to continue to use our system of writing valence structures for molecules, we must adapt the system so that highly delocalized molecules like benzene can be realistically treated. Thus, no single valence structure gives a valid representation of benzene. In using resonance theory to adapt our structural representations to more accurately represent resonance stabilized molecules, we often need to represent such molecules by more than one valence structure:
These valence structures are then called "resonance structures" or "canonical structures", because individually they do not adequately represent the structure of the real compound.As shown in the depiction below, the relationship between the two structures is usually shown by a curved arrow, which depicts the flow of electrons. Bear in mind, that in generating resonance structures, only electrons, not nuclei , are moved. Either electrons in bonds (usually pi bonds) or on atoms may be moved using the curved arrows.
Note that the electrons that are moved in benzene are the electrons of the pi bond (called pi electrons).
THE GEOMETRIC AND ENERGETIC CONSEQUENCES OF RESONANCE STABILIZATION.
Because of its six-fold symmetry, the structure of benzene is often represented with a circle in the middle. Alternately, a single canonical structure is sometimes used for brevity, and this is called a Kekule structure.
ORBITAL PICTURE OF THE CYCLIC OVERLAP IN BENZENE. Note that all six carbons in benzene are trigonally hybridized and the entire molecule is coplanar. Each carbon therefore contributes a 2pz AO to the pi bonding system. A key aspect of this is that each of these orbitals overlaps with two other 2pz AO's on adjacent carbon atoms, one to the left and one to the right, not just to a single one. Thus the system of overlapping orbitals acts as a unit, all electrons being entirely delocalized over the ring. Because overlap is much more extensive, bonding is stronger than in a simple alkene pi bond.
WHEN IS RESONANCE THEORY REQUIRED ? Whenever more than one reasonable valence structure can be written for a species. ( In this case, this normally means that the electrons are more delocalized than can be shown by any one structure).
EXAMPLE 2--THE ACETATE ION. If one examines the reasonable valence structures for the acetate ion (see below), it is evident that, as in the case of benzene, two structures of equivalent energy are available. We can expect that the real structure will be intermediate between the two and that the stability of the ion will be much greater than is implicit in either canonical structure, i.e., the acetate ion is resonance stabilized.
Moving Electrons.In drawing canonical structures, electron are moved by curved electron flow arrows. The electrons may be in pi bonds, as in the case of benzene, or on atoms, as in the case of acetate ion. They can be moved from bonds to other adjacent bonds, from bonds to atoms,or from atoms to bonds. In the acetate ion, electrons are moved from atoms to bonds and from bonds to atoms . In benzene, the electrons are moved from one bond to another.
Resonance stabilization energies are maximized in the following conditions:
1. Two resonance structures of equal energy can be written.
2. The greater the number of equienergetic structures which can be written, the greater the resonance stabilization.
3. If the resonance structures involve a cyclic system of p orbitals, as in the case of benzene (but not acetate ion), resonance stabilization can reach its maximum. These highly stabilized systems are called aromatic systems.
: Another approach to representing highly delocalized systems is to use a single dotted line/partial charge structure. In this context, a dotted line means a partial bond and partial charge is indicated by a d: The DL/PC structure for the acetate ion can be represented as shown below:
The dotted lines mean partial pi bonds. So each CO bond has one sigma bond and a partial pi bond. Both CO bonds are equivalent. The negative charge appears equally on both oxygens (not on carbon).
DERIVATION OF DL/PC STRUCTURES FROM RESONANCE THEORETICAL TREATMENTS. How do we get these dotted line structures. A simple and logical way is to look at both resonance structures. If a bond is full in one and absent in the other, it is partial, and therfore dotted, because the real structure is intermediate between the two structures. If an atom is charged in one structure and not in the other, it is partially charged, and for the same reason. So, the usual procedure is to write the reasonance treatment and then derive the DL/PC structure from this.
BACK TO THE BAULD HOME PAGE